Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6660
Review Article(ISSN: 2637-6660) 
Nanovaccines in aquaculture Volume 2 - Issue 1
Vinay Tharabenahalli Nagaraju*
- ICAR- Central Institute of Brackish water Aquaculture, Chennai, India
Received:July 30, 2019; Published:August 26, 2019
*Corresponding author:Vinay Tharabenahalli Nagaraju,ICAR- Central Institute of Brackish water Aquaculture, Chennai, India
DOI: 10.32474/ANOAJ.2019.02.000129
Abstract
Efficient vaccines and delivery systems are required to prevent and control emerging and re-emerging infectious diseases in aquaculture. The failure is mainly due to the inability to design vaccines evoking appropriate immune responses. The use of nanoparticles has provided a tremendous opportunity to design vaccine delivery systems which are efficient in targeted delivery, providing stability to antigens and act as efficient adjuvants. This review provides an overview of the use of different nanoparticle systems for the delivery of fish vaccines.
Keywords: Nanovaccine, Vaccine delivery, Adjuvant, Fish vaccine
Introduction
Vaccination has had a major impact on control and prevention of infectious diseases in aquaculture Brudeseth, et al. [1] despite that there are many infectious diseases for which the development of an effective vaccine has been difficult to achieve. The vaccine development has had a transition from this conventional method of using whole pathogen to using only the required protein and peptide antigens which have reduced the unwanted side effects, but the immunogenicity of these antigens has gone down drastically Smith, et al. [2]. To enhance the immunogenicity of vaccines, use of adjuvants and efficient delivery systems are very essential Petrovsky and Aguliar, [3]; Corradin and Giudice, [4]; Evensen [5]. ecent research has been focused on the use of nanoparticles (NP’s) as adjuvants and efficient delivery systems in fish vaccine development. Nanoparticles are known to exhibit interesting properties different from their parent material which includes increased relative surface area and quantum size effects. These characteristics of nanoparticles are of great importance in terms of application in medical field Yildirimir et al. [6]. Due to their nano size, nanoparticles can be taken up by cellular endocytosis mechanism Zaman et al. [7]; Zhao et al. [8] which facilitate the cellular uptake of antigens and increase the ability of antigen presentation Oyewumi et al. [9]; Kim et al. [10]; Shaalan et al. [11]. Studies have demonstrated that application of nanotechnology increases solubility, stability, targeting, biocompatibility and permeability of vaccines Frohlich [12]; You et al. [13]; Doll et al. [14]; Lai et al. [15]. Nanovaccines, thus developed are made of nanoparticles formulated with antigens either encapsulated within or adsorbed on to the surface against which an immune response is desired Gregory et al. [16]; Zaman et al. [7]. The advantages of nanovaccines include protection of antigens by encapsulation from degradation, site specific delivery of antigens, enhanced bioavailability and reduced side effects Zolnik et al. [17]; Gregory et al. [16]; Zaman et al. [7]. This review presents an overview of various nanoparticle-based fish vaccines.
Nano-adjuvants and Delivery Systems
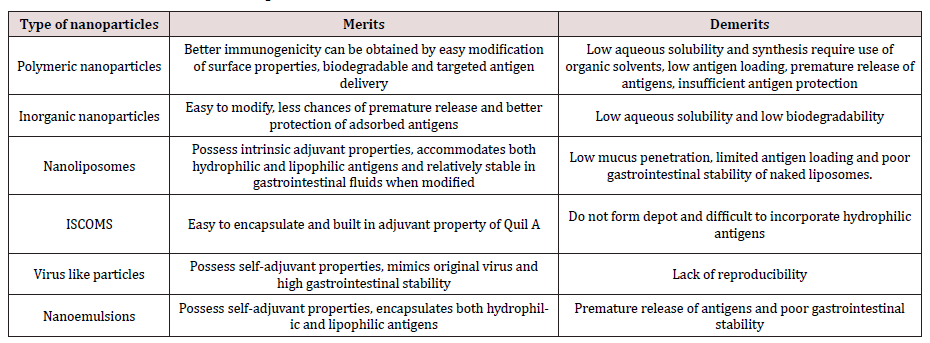
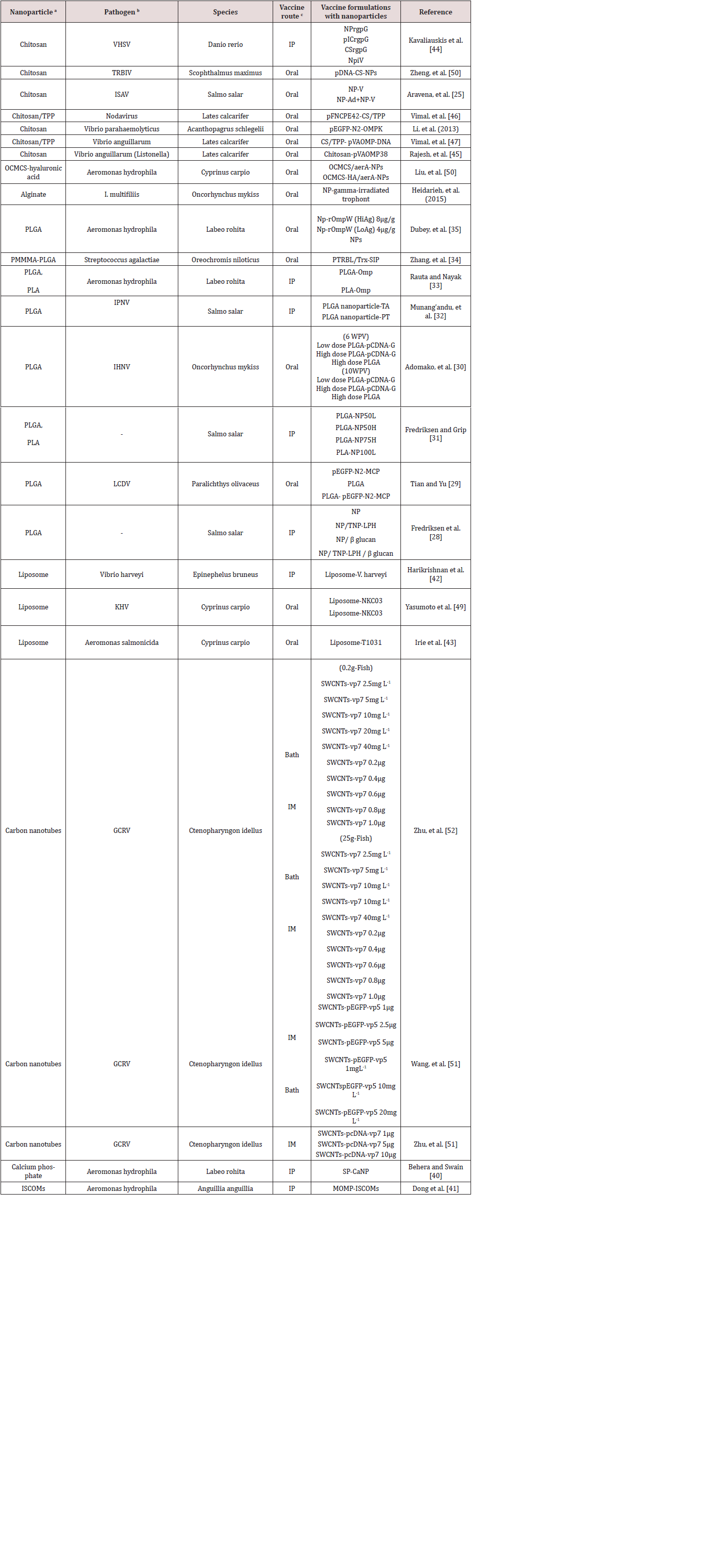
Nanoparticles in vaccine development can be grouped according to their action, either as an efficient mode of delivery system or an adjuvant. Nanoparticles which function as delivery systems will deliver the antigen to targeted immune cells while protecting it and immune potentiating adjuvant nanoparticles will activate a specific pathway which helps in efficient antigen uptake and processing Hølvold et al. [18]; Tafalla et al. [19]. Further, the nanoparticles can be classified as biodegradable or non-biodegradable based on their properties to get decomposed in biological system. In general, the other forms of nanoparticles used in vaccine studies include virus-like particles (VPL’s), nanoliposomes, immunostimulating complexes (ISCOMs), nanoemulsions and metal nanoparticles Gregory et al. [16]; Zhao et al. [8]; Shaalan et al. [11]. Table 1 provides the details of types of nanoparticles applied in vaccine research with their merits and demerits. The type of nanoparticles used in developing fish vaccines have been restricted mainly to polymeric nanoparticles, nanoliposomes, carbon nanotubes, calcium phosphate, ISCOMs and the application of other forms of nanoparticles need to be explored. The present status of nanoparticle-based fish vaccines is summarized in Table 2.
a PLGA: Poly (Lactic-Co-Glycolic Acid), OCMCS: Oleoyl-carboxymethy-chitosan, PMMMA: Poly [(methyl methacrylate)-co-(methylacrylate)- co- (methacrylic acid)], PLA: Poly (Lactic- Acid), ISCOMs: Immunostimulating Complexes. b VHSV: Viral hemorrhagic septecemia virus. TRBIV: Turbot reddish body iridovirus, GCRC: Grass carp reovirus, ISAV: Infectious salmon anemia virus, IPNV: Infectious pancreatic necrosis virus, IHNV: Infectious hematopoietic necrosis virus, LCDV: Lymphocystis disease virus, KHV: Koi herpes virus. c IM: Intramuscular, IP: Intraperitoneal
Inorganic Nanoparticles
Inorganic nanoparticles are used in vaccine research both as adjuvants and potential vaccine delivery systems due to their attractive physical and chemical properties Sahdev et al. [20]; Zhao et al. [8]. There are several inorganic nanoparticles based on carbon, calcium phosphate, gold, silver, silicate, aluminium, titanium etc., among which carbon nanotubes and calcium phosphate are evaluated as vaccine delivery systems in fish vaccines. The inorganic nanoparticles have good adjuvant properties and stabilities but they have certain limitations in their chemistry and physical properties. Due to their varied chemistry polymeric nanoparticles are the widely used nanoparticles in vaccine research. Till date the most explored nanoparticles in fish vaccine studies are the polymeric PLGA and chitosan for administration of viral as well as bacterial antigens.
Polymeric Nanoparticles
The most preferred nanoparticles in vaccine research are the polymeric nanoparticles due to their biodegradable nature, biocompatibility and diverse chemical properties. Polymeric nanoparticles have the capacity to conjugate or encapsulate any antigens within itself or on their surface Marasini et al. [21]; Sahdev et al. [20]. There are several polymeric nanoparticles which can be grouped based on their origin as: Naturally derived and synthetically derived polymers.
Naturally Derived Polymers
Chitosan is a naturally derived biodegradable polymer and is extracted from various chitinous materials mainly from the exoskeleton of crustaceans and hence it can be earmarked as a green nanoparticle. It is highly abundant, biodegradable and biocompatible, making it an attractive candidate for vaccine delivery Sahdev et al. [20]. Hyaluronic acid (HA) is a natural polymer composed of D-glucuronic acid and N-Acetyl-Dglucosamine and is a component of cartilaginous tissue Sahdev et al. [20]; Smith et al. [2]. It also plays an important role in immune response by modulating leukocyte trafficking Mummert, [22]; Sahdev et al. [20]. It is biocompatible, biodegradable, hydrophilic and due to high abundance in nature and makes it as one of the attractive candidate nanoparticles for vaccine delivery Sahdev et al. [20]; Smith et al. [22]. Alginate is an extract of naturally available brown algae and also it can be found as a polysaccharide in some bacteria. It is made of repeated units of unbranched polyanionic polysaccharides α-L-guluronic acid and β-D-mannuronic acid Ji et al. [23]. It is biodegradable, biocompatible, non-toxic, acid resistant, mucoadhesive and most suited for oral vaccine delivery Wee and Gombotz, [24]; Aravena et al. [25].
Synthetically Derived Polymers
Poly (Lactic-Co-Glycolic Acid) (PLGA) is a synthetic copolymer of lactic acid and poly glycolic acid. It is a very commonly used delivery system in biomedical research. The use of PLGA is approved by US-FDA and European Medicine Agency (EMA) due to its biocompatibility, non-toxicity and highly biodegradable nature. Upon administration it undergoes hydrolysis and release glycolic and lactic acids which are eventually removed from body by citric acid cycle Panyam and Labhasetwar [26]; Sahdev et al. [20]; Ji et al. [23]. Poly (Lactic-Co-Glycolic Acid) is also used as an adjuvant, alternative to alum for prolonging the in-vivo antigenic exposure time Toita et al. [27]; Smith et al. [2]. It is in general used for the controlled release of nucleic acids, proteins and peptides and hence it is the most explored nanoparticle for the delivery of fish vaccines Fredriksen et al. [28]; Tian and Yu [29]; Adomako et al. [30]; Fredriksen and Grip [31]; Munang’andu et al. [32]; Rauta and Nayak, [33]; Zhang et al. [34]; Dubey et al. [35]. Poly (lactic acid) (PLA) is a synthetic polymer comprising of repeated lactide monomers that degrades into biocompatible lactic acid. It is less degradable compared to PLGA and hence has a limited usage as a vaccine delivery system Smith et al. [2].
Lipid Based Biomolecular Nanoparticles
Several biomolecule-based nano-formulations are used extensively in vaccine research such as liposomes, ISCOMs, micelles and virus-like particles. Among these, liposomes and ISCOMs are used for fish vaccine delivery Kim et al. [10]. Nanoliposomes have been well documented for their diverse ability to deliver various hydrophilic and hydrophobic antigens as they possess hydrophilic head and hydrophobic tail Ji et al. [24]; Smith et al. [2]. These are formed by non-toxic and biodegradable self-assembled structures of phospholipids consisting of an internal aqueous core entrapped by a lipid bilayer Zhao et al. [8]. Surface modification of liposomes is easy, and it can increase the immunogenicity to enhance both humoral and cell-mediated immunity Kim et al. [10]. Immuno-stimulating complexes (ISCOMs) are self-assembled cage like structures usually of 40 nanometer size and consisting of cholesterols, phospholipids and Quil A saponin. The cage like structures helps in entrapping the antigens or adjuvants. Immuno stimulating complexes are good antigen carriers and they themselves are very efficient adjuvants as they are formed of saponin Marasini et al. [21]. Immuno stimulating complexes are researched for more than 3 decades now and are restricted to veterinary use due to the mild toxic effects having hemolytic properties Sjolander et al. [36].
Biosafety Concerns of Nanoparticle Toxicity
While the nanoparticles have shown the undisputable potential for their wide range of applications, the very nature which make them interesting might have negative effects as well Elsaesser and Howard [37]; Gregory et al. [38]; Zellner [39]. Since, they can cross the blood brain barrier (BBB), the applications have to be made carefully as it may cause serious troubles Yildirimir et al. [6]. The evaluation of nanoparticle toxicity is not easy and cannot be predicted based on the toxicity profile of their parent material as they exhibit different properties and are also taken up by cells in an entirely different way as compared to their parent materials. Recent study focused on understanding the mechanism of nanoparticle toxicity suggests the toxicity may range from cell necrosis to reactive oxygen species (ROS) induced apoptosis Elsaesser and Howard [37].
Conclusion and Future Prospects
In the last decade there has been a remarkable advancement in nanotechnology and its application in biomedicine especially in vaccine delivery [38-45]. Nanovaccines developed for aquacultured species has a fair share in this advancement. Nanoparticles have shown to enhance the immunogenicity of weak antigens and they provide many advantages over conventional adjuvant approaches like having better release kinetics, stability and targeted delivery. Given the nature of aquaculture the most preferred route of vaccination is oral delivery as it is not practical to inject every fish unlike other terrestrial species [45-50]. Nanoparticles provide an opportunity to design vaccines which have gastrointestinal stability, a major requirement for oral vaccines [51,52].
Acknowledgement
This work was supported by Indian Council of Agricultural Research (IIAB-FHM-01-01).
References
- Brudeseth BE, R Wiulsrod, BN Fredriksen, K Lindmo, KE Lokling, et al. (2013) Status and future prospects of vaccines for industrialized fin-fish farming. Fish Shellfish Immunol 35(6): 1759-1768.
- Smith JD, LD Morton, BD Ulery (2015) Nanoparticles as synthetic vaccines. Curr Opin Biotechnol 34: 217-224.
- Petrovsky, NJC Aguliar (2004) Vaccine adjuvants: Current state and future trends. Immunol Cellbiol 82(5): 488-496.
- Corradin G, GD Giudice (2005) Novel Adjuvants for Vaccines. Current Medicinal Chemistry-Anti-Inflammatory & Anti-Allergy Agents 4: 185-191.
- Evensen Ø (2009) Development in fish vaccinology with focus on delivery methodologies. adjuvants and formulations. The use of veterinary drugs and vaccines in Mediterranean aquaculture (39): 177-186.
- Yildirimer L, NT Thanh, M Loizidou, AM Seifalin (2011) Toxicological considerations of clinically applicable nanoparticles. Nanotoday 6: 585-607.
- Zaman M, MF Good, I Toth (2013) Nanovaccines and their mode of action. Methods 60(3): 226-231.
- Zhao L, A Seth, N Wibowo, CX Zhao, N Mitter, et al. (2014) Nanoparticle vaccines. Vaccine 32(3): 327-337
- Oyewumi MO, A Kumar, Z Cui (2010) Nano-microparticles as immune adjuvants. correlating article sizes and the resultant immune responses. Exp Rev Vac 9(9): 1095-1107.
- Kim MG, JY Park, Y Shon, G Kim, G Shim, et al. (2014) Nanotechnology and vaccine development. Asian J Pharma Sci 9(5): 227-235.
- Shaalan M, M Saleh, M El-Mahdy, M El-Matbouli (2016) Recent progress in application of nanoparticles in fish medicine: A review. Nanomed Nanotechnol 12(3): 701-710.
- Frohlich E (2012) The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomed 7: 5577-5591.
- You C, C Han, X Wang, Y Zheng, Q Li (2012) The progress of silver nanoparticles in antimicrobial mechanism. clinical application and cytotoxicity Mol Biol Rep 39(9): 9193-9201.
- Doll TA, S Raman, R Dey, P Burkhard (2013) Nanoscale assemblies and their biomedical applications. J R Soc Interface 10(80): 20120740.
- Lai W, Z Hu, Q Fang (2013) The concerns on biosafety of nanomaterials. JSM Nanotech Nanomed 1: 1009.
- Gregory AE, R Titball, D Williamson (2013) Vaccine delivery using nanoparticles. Front Cell Infect Mi 3:
- Zolnik BS, AG Fernandez, N Sadrieh, MA Dobrovolskaia (2010) Nanoparticles and immune systems. Endocrinology 151(2): 458-465.
- Hølvold LB, FN Børge, B Jarl, RA Dalmo (2013) Transgene and immune gene expression following intramuscular injection of Atlantic salmon (Salmo salar L.) with DNA-releasing PLGA nano- and microparticles. Fish Shellfish Immunol 35(3): 890-899.
- Tafalla C, J Bøgwald, RA Dalmo (2013) Adjuvants and immunostimulants in fish vaccines: current knowledge and future perspectives. Fish Shellfish Immunol 35(6): 1740-1750.
- Sahdev P, LJ Ochyl, JJ Moon (2014) Biomaterials for nanoparticle vaccine delivery systems. Pharma Res 31(10): 2563-2582.
- Marasini N, M Skwarczynski, I Toth (2014) Oral delivery of nanoparticle-based vaccines. Exp Rev Vac 13(11): 1361-1376.
- Mummert ME (2005) Immunologic roles of hyaluronan. Immunol Res 31(3): 189-206.
- Ji J, D Torrealba, A Ruyra, N Roher (2015) Nanodelivery systems as new tools for Immunostimulant or vaccine administration. targeting the fish immune system Biology 4(4): 664-696.
- Wee S, WR Gombotz (1998) Protein release from alginate matrices. Adv Drug Deliv Rev 31(3): 267-285.
- Aravena AR, AM Sandino, E Spencer (2013) Nanoparticles and microparticles of polymers and polysaccharides to administer fish vaccines. Biol Res 46(4): 407-419
- Panyam J, V Labhasetwar (2003) Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev 55(3): 329-347.
- Toita R, Y Kanai, H Watabe, K Nakao, S Yamamoto, et al. (2013) Biodistribution of (125) I-labeled polymeric vaccine carriers after subcutaneous injection. Bioorg Med Chem 21(17): 5310-5315.
- Fredriksen BN, K Saevareid, L McAuley, ME Lane, J Bogwald, et al. (2011) Early immune responses in Atlantic salmon (Salmo salar L.) after immunization with PLGA nanoparticles loaded with a model antigen and β-glucan. Vaccine 29(46): 8338-8349.
- Tian J, J Yu (2011) Poly (lactic-c0-glycolic acid) nanoparticles as candidate DNA vaccine carrier for oral immunization of Japanese flounder (Paralichthys olivaceus) against lymphocystis disease virus. Fish Shellfish Immunol 30(1): 109-117.
- Adomako M, S St-Hilaire, Y Zheng, J Eley, RD Marcum (2012) Oral DNA vaccination of rainbow trout, Oncorhynchus mykiss (Walbaum), against infectious haematopoietic necrosis virus using PLGA [Poly (D, L-Lactic-Co-Glycolic Acid)] nanoparticles. J Fish Dis 35(3): 203-214.
- Fredriksen BN, J Grip (2012) PLGA/PLA micro-and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of Atlantic salmon (Salmo salar L.). Vaccine 30(3): 656-667.
- Munangandu HM, BN Fredriksen, S Mutoloki, B Brudeseth, TY Kuo, et al. (2012) Comparison of vaccine efficacy for different antigen delivery systems for infectious pancreatic necrosis virus vaccines in Atlantic salmon (Salm salar L.) in a cohabitation challenge model. Vaccine 30(27): 4007-4016.
- Rauta PR, B Nayak (2015) Parenteral immunization of PLA/PLGA nanoparticle encapsulating outer membrane protein (Omp) from Aeromonas hydrophila. evaluation of immunostimulatory action in Labeo rohita (rohu) Fish Shellfish Immunol 44(1): 287-294.
- Zhang L, Z Zeng, C Hu, SL Bellis, W Yang, et al. (2015) Controlled and targeted release of antigens by intelligent shell for improving applicability of oral vaccines. Biomaterials 77: 307-319.
- Dubey S, K Avadhani, S Mutalik, SM Sivadasan, B Maiti (2016) Aeromonas hydrophila OmpW PLGA nanoparticle oral vaccine shows a dose-dependent protective immunity in rohu (Labeo rohita). Vaccines 4(2): 21.
- Sjolander A, JC Cox, IG Barr (1998) ISCOMs: an adjuvant with multiple functions. J Leuk Biol 64(6): 713-723.
- Elsaesser A, CV Howard (2015) Toxicology of nanoparticles. Adv drug del Rev 64(2): 129-137.
- Gregory Zellner R (2015) Biological responses to nanoscale particles. Beilstein J Nanotech 6: 380-38
- Aravena AR, Y Fuentes, J Cartagena, T Brito, V Poggio, et al. (2015) Development of a nanoparticle-based oral vaccine for Atlantic salmon against ISAV using an alphavirus replicon as adjuvant. Fish Shellfish Immunol 45(1): 157-166.
- Behera T, P Swain (2011) Antigen adsorbed calcium phosphate nanoparticles stimulate both innate and adaptive immune response in fish. Labeo rohita H Cell Immunol 271(2): 350-359.
- Dong CF, TL Lin, H Gong, YSD Ou, S Yang (2005) Major outer membrane protein (MOMP) of Aeromonas hydrophila induced protective immunity to European eels (Anguilla anguilla). Acta Hydrobiol Sin 29: 285-290.
- Harikrishnan R, JS Kim, C Balasundaram, MS Heo (2012) Vaccination effect of liposomes entrapped whole cell bacterial vaccine on immune response and disease protection in Epinephelus bruneus against Vibrio harveyi. Aquaculture 342-343: 69-74.
- Irie T, S. Watarai, T Iwasaki, H Kodama (2005) Protection against experimental Aeromonas salmonicida infection in carp by oral immunization with bacterial antigen entrapped liposomes. Fish Shellfish Immunol 18(3): 235-242.
- Kavaliauskis A, M Arnemo, M Speth, L Lagos, AL Rishovd, et al. (2016) Protective effect of recombinant VHSV-G vaccine using poly (I:C) loaded nanoparticles as an adjuvant in zebrafish (Danio rerio) infection model. Dev Comp Immunol 61: 248-257.
- Rajesh SK, VPI Ahmed, V Parameswaran, R Sudhakaran, VS Babu, et al. (2008) Potential of chitosan nanoparticles for oral delivery of DNA vaccine in Asian sea bass (Lates calcarifer) to protect from vibrio (Listonella) anguillarum. Fish Shellfish Immunol 25(1-2): 47-56.
- Vimal S, AS Majeed, KSN Nambi, N Madan, MA Farook, et al. (2014) Delivery of DNA vaccine using chitosan-tripolyphosphate (CS/TPP) nanoparticles in Asian sea bass. Lates calcarifer (Bloch,1790) for protection against nodavirus infection Aquaculture 420-421: 240-246.
- Vimal S, G Taju, KSN Nambi, SA Majeed, VS Babu, et al. (2012) Synthesis and characterization of CS/TPP nanoparticles for oral delivery of gene in fish. Aquaculture 358-359: 14-22.
- Wang Y, GL Liu, DL Li, F Ling, B Zhu, et al. (2015) The protective immunity against grass carp reovirus in grass carp induced by a DNA vaccination using single-walled carbon nanotubes as delivery vehicles. Fish Shellfish Immunol 47(2): 732-742.
- Yasumoto S, Y Kuzuya, M Yasuda, T Yoshimura, T Miyazaki (2006) Oral immunization of common carp with liposome vaccine fusing koi Herpesvirus antigen. Fish Pathol 41: 141-145.
- Zheng F, H Liu, X Sun, Y Zhang, B Zhang (2016) Development of oral DNA vaccine based on chitosan nanoparticles for the immunization against reddish body iridovirus in turbots (Scophthalmus maximus). Aquaculture 452: 263-271.
- Zhu B, GL Liu, YX Gong, F Ling, GX Wang (2015) Protective immunity of grass carp immunized with DNA vaccine encoding vp7 gene of grass carp reovirus using carbon nanotubes as carrier molecule. Fish Shellfish Immunol 42(2): 325-334.
- Zhu B, GL Liu, YX Gong, F Ling, LS Song, et al. (2014) Single walled carbon nanotubes as candidate recombinant subunit vaccine carrier for immunization of grass carp against grass carp reovirus. Fish Shellfish Immunol 41(2): 279-293.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...