Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6660
Research Article(ISSN: 2637-6660) 
Investigation of Sorption of Naphthalene Derivatives by Mg-Al-Layered Double Hydroxides Volume 2 - Issue 1
Butenko E1* and Guegan R2
- 1State Technical University, Mariupol, Ukraine
- 2Institut des Sciences de la Terre d’Orléans, France
Received: April 24, 2019; Published:July 17, 2019
*Corresponding author:Butenko E,State Technical University, Mariupol, Ukraine
DOI: 10.32474/ANOAJ.2019.02.000126
Introduction
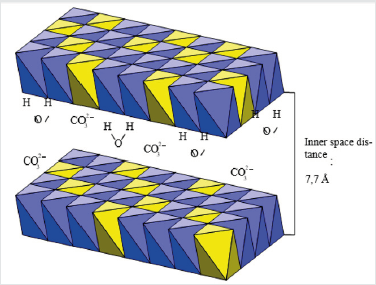
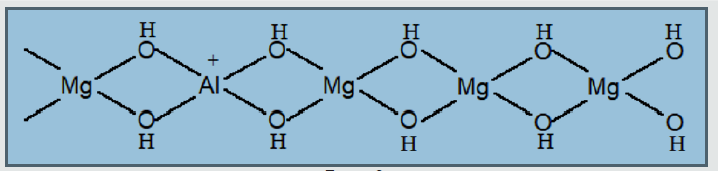
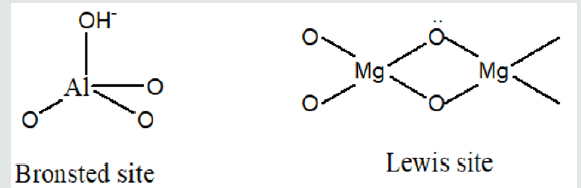
Layered double hydroxides find application as sorbents, catalysts, supported of catalytic spices. Their use is especially effective as sorbents of organic compounds from a liquid phase. In particular, they are the active absorbers of aromatic and naphthalene derivatives from a liquid phase. Development of effective methods of adjusting of processes of activating and modification of mineral sorbents with the purpose of increase of degree of cleaning of sewages has an important theoretical and applied value [1]. Our work is devoted to researches of processes of sorption of naphthalene and his derivatives by layered double hydroxides. This work includes the researches of structural and adsorption descriptions of layered double hydroxides, with a purpose to adjusting of processes of their activating and modification. Layered double hydroxides are the products of isomorphic substitution of cations of metals in hydroxides on cations of more high oxidation degree (Figure 1). The isomorphic substitution of ions of Mg2+ on Al3+ cations in a brucite results in the origin of surplus positive charge, and, consequently, to basic properties, therefore layered double hydroxides possess anion-exchange properties (Figure 2). Bronsted basic site in such compounds can be hydroxyl localized on a tetrahedron aluminum. Lewis basic sites are the undivided electronic pair of oxygen (Figure 3). The presence of basic sites of Bronsted and Lewis types enables the process of anionic ex-change in internal space of layered double hydroxides. Inner space distance in layered double hydroxides depends mainly on nature of anions and solvates molecules in the internal space of layered double hydroxides, and also from the degree of isomorphic substitution. At the sorption of organic molecules with branched radical, the main parameter, determining inner space distance in layered double hydroxides, is the size of hydrocarbon radical and his orientation in the inner space
Experimental
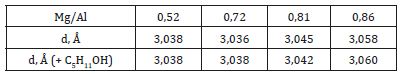
Layered double hydroxides of general formula of MgxAly(OH)z of variable composition were got by the method of besieging from proper solutions of Mg(NO3)2 and Al(NO3)3 salts. Filtered no less than 18 hours. Dried at the temperature of 80оС at 96 hours. The samples with 0,52; 0,72; 0,81; 0,86 mol/mol of MgO were obtained. For initial layered double hydroxides, the specific surface was determined, and X-ray analysis was conducted. The sorption researches of naphthalene and β-naphthol by layered double hydroxides were conducted. Layered double hydroxides of different composition were put into 1% solutions of naphthalene and β-naphthol and abandoned on days. Solutions were filtered, and sorbents dried out at 1250С.
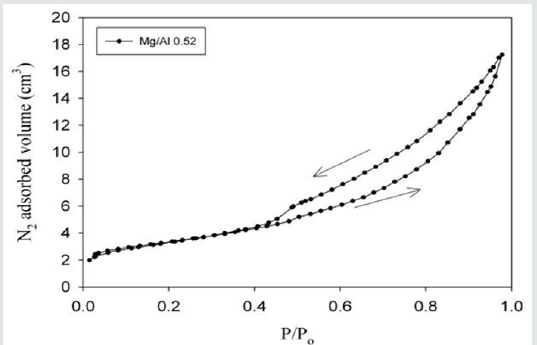
The specific surface was determined by the BET method. In order to realize the N2 adsorption and desorption isotherms, about 150 mg of LDHs were first dried at 110ºC during 24 hours under a vacuum of 10-3mbar. The experiences were performed on Nova Surface Analyzer from Quantachrome Instruments. Specific surface areas of both LDHs were determined using the multi-point BET method (Figure 4). Nitrogen vapor adsorption data at 77K were obtained for relative vapor pressures (P/Po) from 0.013 to 0.0973. The cross-sectional area of a nitrogen molecule was assumed to be 16.2Ų. The adsorption and desorption isotherms are presented in the next figures, where P/Po represents the relative vapor pressures and V the N2 adsorbed/desorbed volume. The behavior of the ad-sorption isotherms is from type II, using the classification by IUPAC (IUPAC, 1985), and shows unrestricted monolayermultilayer adsorption. The XRD patterns were collected on an ARL X’TRA Diffractometer (Thermo Electron Corporation, Ecublens, Switzerland) operating at 40kV and 40 mA with CuKα radiation (l~1.54Å). The patterns were recorded in the 2-theta (2Θ) range from 1 to 64°, in steps of 0.05° and counting time of 5s per step. Samples crushed using an agate mortar and a pestle and then directly put into the sample holder.
Results and Discuss
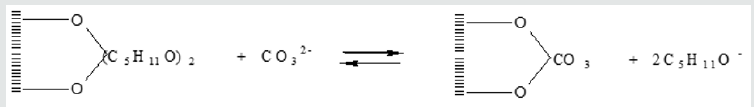
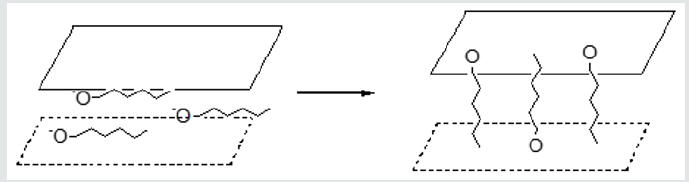
The preliminary experiments on the change of interlayered distances in layered double hydroxides after the sorption of organic molecules were conducted with the use of alcohols. Research of sorption of amyl alcohol showed that alcohols reacted with layered double hydroxides in the reaction of anionic exchange, with establishment of equilibrium. For proof of such process the sorbent after adsorption was washed of ethyl alcohol and ether to absence of tracks of amyl alcohol, and then was washed by water solution, containing carbonate-ions. In finished solution the alcohol was found by chromatographic method, that talks about undergoing of next reaction (Figure 5). The time of establishment of equilibrium depends on the rate of process of intercalation of alcohols in the inter layered space with the simultaneous moving apart of layers and re-orientation of hydrocarbon radicals. In initial moment the hydrocarbon radicals’ bed parallel to the matrix layers. For confirmation of this supposition the structure of layered double hydroxides was studied. Re-searches of structure of layered double hydroxides after the sorption of amyl alcohol are shown in Table 1. For the protracted staying of sorbents in alcohols, especially at an enhance able temperature, there is an increase of interlayered distance. At increase of length of hydrocarbon radicals, with achievement of number of atoms of carbon equal 5, there is an increase of interlayered distance (Figure 6). Investigating the sorption of naphthalene derivatives, we discovered that at the protracted stay of sorbents in solutions, containing these compounds, the degree of sorption increases. There was a supposition, that moving of inorganic layers under the action of organic molecules is a reason of it. The purpose of our work was to research the processes of physisorption of naphthalene and anionic exchange of β-naphthol on layered double hydroxides of variable composition, and also to study the changes in a structure, basicity and sizes of internal space because of sorption processes.
A sorption of naphthalene is a process of physisorption, and β-naphthol participates in an anionic exchange. β-naphthol is a weak acid, so the process of sorption takes place considerably quickly, then for alcohols. Adsorption of organic compounds on layered double hydroxides, depending on the structure of organic molecule, can takes place both as the process of physisorption and as ionic exchange. In first cases activity and selectivity of process of sorption is determined the specific surface of sorbent and size of pores, in the second one by the concentration of active sites on the surface of sorbent and their availability for the substrate molecules. At an anionic exchange the inner space distance of layered double hydroxides can change in wide limits, at physical adsorption it does not observed, however, to our opinion, this process can take place also. Influence of temperature on a sorption from water solutions not simply. At the sorption on the microporous sorbents of substances, the sizes of which molecules are near of the effective sizes of pores, the penetration of these molecules in pores depends on their kinetic energy. At sufficient energy of molecule of sorbate get to the windows of pores; otherwise there is only insignificant absorption on the surface of mezo- and macropores. By other words, a sorption capacity rises with growth of temperature. At the same time the physisorption, as any exothermic process, gets worse with growth of temperature. Therefore, total influence, fixing these two phenomena of active and physical adsorption, can have maximum at a certain temperature. In the beginning the process of sorption undergoing quickly due to the positively charged sur-face of adsorbent. Further the process of adsorption flows slowly, probably, due to an electrostatic hindrance between the negatively charged anions on the surface. For the estimation of distinction in a physisorption and ionic exchange the experiments on adsorption β-naphthol (anionic exchange) and naphthalene (physisorption) were carried out. The measuring of sizes of internal space were carried out with X-ray analysis. The results of experiments are showed on these pictures (Figure 7). On the whole, all three pictures are very alike, a structure changes insignificantly. There is a change of intensity and positions of one peak at 11,8 degrees. On the selected insertion intensity falls, and a peak is slightly displaced in the area of less corners. An undent means the increase of interlayered distance which corresponds this peak. Because of this peak corresponds with d001 parameter, it talks about penetration of organic compound into the inner space, that is also confirmed that peaks at 43, 61 and a 62 degree are alike displacements. Because of the peak, correspond to β-naphthol, changes variously: grows somewhere, and falls somewhere, so that it is a not change of stake of phase, and, rather, distortion of lattice.
Figure 7: X-Ray diffraction patterns of naphthalene-LDH, beta naphtole-LDH composites and bulk LDH for the composition Mg to Al of 0.52.
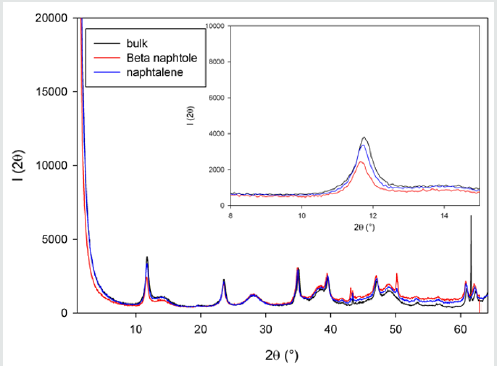
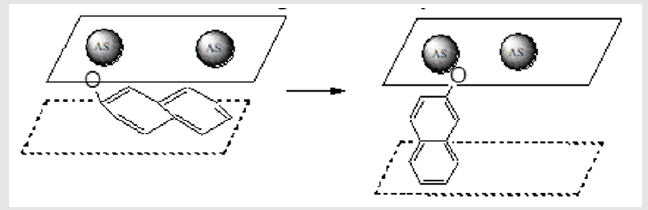
There is a change of structure because of there is displacement of a few peaks, appear and grow on intensity new peaks in area of 50 degrees (initial ◊ naphthalene ◊ β-naphthol), all of it takes place at unchanging position and intensity of other peaks (that, it is not the error of experiment). From the presented X-ray pictures evidently, that maximal rejections are observed at the sorption of β-naphthol. Possible reason of it is that the aromatic rings of the adsorbed naphthalene bed parallel to the inorganic layers, regardless of concentration of active centers. Co-operating of π-electrons of aromatic rings with the layers of matrix causes the change of interlayered distance (Figure 8). The size of interlayered distance is about 7Å. This size is comparable with the «thickness» of aromatic ring, making 3,5Å, while a diameter of molecule of benzene is 7,1Å. And for a phenol and β-naphthol a trivial-parallel location is observed only in the initial stage of ionic exchange. Effective area β-naphthol is about 9,89Å2, that considerably less size of one active site. The equilibrium, attended with the increase of interlayered distance and increase of number of active centers accessible for next molecules of β-naphthol, is set in future [2]. This process is accompanied the increase of specific surface of layered double hydroxides (Table 2). Thus, in our work the distinction of properties of layered double hydroxides at the sorption of organic compounds on an ion-exchange mechanism and at a physical sorption was shown.
References
- Butenko E (2018) Use of layered double hydroxides to create new environmental technologies. Int J Sci Res Environ Sci 23(1-2): 137-142.
- Butenko E, Kapustin A, Malyshev A (2014) LDHs as adsorbent of the phenols and their environmental application. American Journal of Environmental Protection 2(1): 11-15.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...