Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6660
Research Article(ISSN: 2637-6660) 
Formation of Silver Nanoparticles in the root extract of Scutellaria baicalensis and their characterization Volume 1 - Issue 5
Ahmadov IS*, Ramazanov MA, Eyvazova GM, Huseynli SZ and Aliyeva ST
- Department of chemical physics of nanomaterials, Baku State University, Azerbaijan
Received: December 13, 2018; Published: February 04, 2019
*Corresponding author: Ahmadov IS, Department of chemical physics of nanomaterials, Baku State University, Azerbaijan
DOI: 10.32474/ANOAJ.2018.01.000122
Abstract
This research about on the formation of silver nanoparticles (AgNPs) in extract of Scutellaria baicalensis roots. Comprehensive analyzes were carried out and the characteristics of these nanoparticles, which can be used in medical practices and environmental cleaning problems. The roots extracts of Scutellaria baicalensis advocates as reductant and stabilizer. The first proof of the formation of AgNPs is the change of color of solution AgNO3 which was added root extract. Then by UV-Vis spectroscopy identificated the absorption peak of AgNPs at 414nm –461nm interval. Other characterization of AgNPs received with SEM analysis, From SEM pictures it is clear that the AgNPs have a spherical form, with size ranging from 7.12nm to 18.8nm. An average size of AgNPs are 11.35nm. By FTIR analysis of AgNPs was established the structures, the respective bands of the synthesized nanoparticles, and the stretch of bonds.
Keywords: Scutellaria baicalensis; Biosynthesis; Silver nanoparticles; Plant extracts; UV-vis spectra; FT-İR spectra
Introduction
Last years, scientists have tried to create different methods and technologies to get non-toxic forms of nanoparticles. A chemical synthesis of nanoparticles, especially precious metals is widespread. However, since nanoparticles obtained in this way are expensive and toxic, their synthesis in biological methods is of great interest recently. In biosynthesis of nanoparticles no needs applied high pressure and energy, temperature and toxic chemicals. The possible ecofriendly alternatives to chemical and physical methods are biological methods synthesis of nanoparticle using microorganisms [1-3] enzymes [4], fungus [5], and plants or plant extracts [6-8]. Biological synthesis of nanoparticles using plants extracts in many cases is advantageous than other biological methods which may eliminating the elaborate processes in the technological point of view [9]. The application of nanotechnology in medicine has put this issue more seriously. In this regard, silver nanoparticles having a unique optical and mechanical properties, having antiseptic properties, are of particular importance. There are data that AgNPs with size of 1-4nm can directly pass the cell membrane.Such AgNPs in this dimensions and in spherical form play an important role not only in the medical practice but also in the solution of issues such as treatment problems, as well as diagnostics, and sensors. Therefore the synthesis of AgNPs with this sizes one of the major issues in green synthesis of nanoparticles [10] At present, the size of used nanoparticles as the drug carrier is at least 5-10nm, and there is a need for highly clean, non-toxic nanoparticles to increase the efficacy of the combined drugs. Since silver nanoparticles often paid for this demand, their acquisition became more extensive. The practical properties of AgNPs allow applied them in high contrast imaging method and in detection of human serum antibody [11], for the prevent infection against burn and open wounds by topical ointments [12], membrane transport in native microbial cells [13], enhanced IR absorption spectroscopy [14], mass spectrometry of peptides [15], bio-labeling, optical imaging of cancer [16], biosensors for detection of herbicides [17], and glucose sensor for medical diagnostics [18], colorimetric sensor for histidine [19], determination of “brinogens” in human plasma [20], for measuring ammonia concentration by colorimetric sensors [21]. Besides of above, AgNPs have various important applications: for example, they are using as coatings for solar energy absorption, as an intercalation material for electrical batteries, and as optical receptors for biolabeling [22-24].
When synthesizing nanoparticles in plant extracts, three different steps can be distinguished [25,26]. In the first step - socalled - induction phase - occurs a rapid metallic ion reduction and seeds metallic nucleation. In second fase small crystals spontaneously aggregate and transform into large aggregates. In the third - termination phase take place the formation of nanoparticles, the sizes and shapes of the nanoparticles become energetically favourable. In this fase specific biomolecules act as capping agents and stabilized the nanoparticles. This process similar to biomineralization, but the nature of formation nanoparticles in the plant extracts is not yet understood in any depth. [27].
In this work was used plant, well-known in medical practize - Scutellaria baicalensis for the synthesis of AgNPs. Root and leafs of Scutellaria baicalensis have many powerful antioxidants. These antioxidants may take play as reductant during reduction of silver ions into nanoparticles. In addition, the molecules of these antioxidants can be adsorbed or even attached to the surface of the nanoparticles during their formation. Such nanochastists can act as nano drugs. The specific antioxidants on the surface of AgNPs makes possible the future use of AgNPs as a nano-drug. In this experiments, the problems of combining antioxidants with the surface of AgNPs were studied also.
Materials and Methods
Scutellaria baicalensis Georgi - Baikal skullcap have long been used in traditional medicine. This plants is one of species of Scutellaria which has 300 species [28,29]. Because of the high content of flavonoids, such as baicalin and baicalein Scutellaria baicalensis is the most studied specie in this group. The flavonoids which is isolated from S. baicalensis leafs and from roots have a wide range of pharmacological appling and are bioactiv molecules [30,31]. Now the extracts from S. baicalensis widly use in medicine of Russia, China, and other many countries. There are data that the roots of Baikal skullcap has very higher content of baicalin and baicalein compared to other organs. Therefore the roots of Baikal skullcap is more interesting for such purposes. Currently, the skullcap roots are more harvested and used for baicalin and bacalein production. Extracts from root of this plants are using as pharmaceuticals for the many diseases. In some results of researchers, shows however that the shoot extract also had a greater biological activity.
Scutellaria baicalensis root and leaves with flowers was given by Aida Bandaliyeva senior teacher of Department of Pharmaceutical Technology and Management of Azerbaijan Medical University (Figure 1).
Preparation of the scutellaria extracts
The 100g dried root and 50g leaves of the plant of the Scutellaria baicalensis were first ground into a blender. In first variant of experiments 1.6g from powder of root were taken and dissolved in 100ml of distilled water. The received solution was boiled at 100 °C with for 10 minutes keeping the volume in balance and cooled in a refrigerator for 24 hours. For the synthesize AgNPs, a solution of 5.10-3 M AgNO3 was used. Have been taken a 50ml root extract as reductant and added to 450 ml of AgNO3 solution volume. The resulting solution was left for 24 hours in room temperature. Such kind of extract was made also from leaves powder. In the second variant, for the preparation of extract was taken 0.5g Scutellaria root powder and dissolved in 200ml bidistilled water. The solution was keeped for 3 hours at 90 °C. After 3 hours was filtered and added 70% ethanol. This solution for 12 hours was keeped at 4 °C temperature.
Synthesis of silver nanoparticles
For the synthesis of Ag nanoparticles from each samples of extracts which are made in different composition have been taken 50ml and was mixed with 450ml 5.10-3 M aqueous AgNO3. After addition of extract of root to the silver nitrate solution the white color of solution was turned into brown an occurs the formation of AgNPs from AgNO3.
Identification of Ag nanoparticles
Formation of AgNPs was initially characterized by standard protocols such as UV–Visible spectra (Analytic Jena Specord- 250 plus spectrophotometer). In UV-vis spectrometer the light was absorbed and scattered by the sample in the interval of 190nm to 800nm. For the FTIR analysis have been used Varian 3600 FT-IR spectrometer. The solution of silver nanoparticles (sample) firstly was centrifuged and than dried in water bath and were ground with KBr and then made into pellets. These pellets were analyzed by Fourier infrared wavelength varying from 400 to 4000cm-1. The morphology of dried AgNPs was characterized by Scanning Electron Microscope (SEM) - JSM 7600F, JEOL. The sample was placed on the metal disc with vacuum pressure. In SEM analysis have been made element analysis also.
Results and Discussion UV-Vis Spectroscopy
Figure 2: The color change of solution during formation of silver nanoparticles : 1- solution of AgNO3, 2 - extract from the root of Scutellaria baicalensis, 3 - Ag nanoparticle solution.

Figure 3: The UV-vis spectra of Ag nanoparticles synthesized by the root extracts of Scutellaria baicalensis: 1- after 24 hours; 2 – after 15 days; 3-after 1 month by the extract which was prepared into the first variant methods; 4- after 24 hours; 5- after 15 days; 6- after 1 month by the extract which was prepared into the second variant methods.
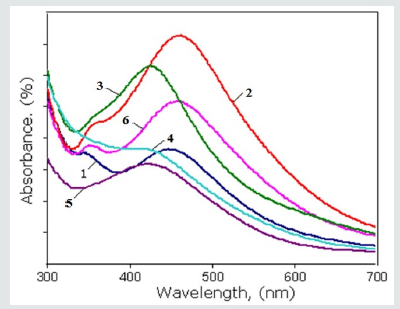
After the adding root extract of Scutellaria to the aqueous solutions of silver nitrate (AgNO3) the formation of AgNPs occurs by inducing the reduction of Ag2+ ions into Ag0 which followed change color of solution. A few hours after the addition of the root extract C to the solution of F, the color of the solution changes and it becomes dark brown due to the surface plasmon resonance phenomenon (Figure 2). In our experiments the reduction was completed after 24 hours with the appearance of brownish - black color which confirms the formation of silver nanoparticles. One of the most widely used method for the identification of nanoparticle formation is UV–Vis spectroscopy which is simple and sensitive techniques. In order to identification of AgNPs, the absorption spectra of synthesized AgNPs were recorded. The UV – vis analysis was studied after through the time duration of 24 hours, 15 days and 1 month. Based on the absorption spectra, the biosynthesized Ag nanoparticles by root extract showed surface plasmon resonance [32] bands at about 414nm–461nm interval as shown in (Figure 3). İt is interesting that on increasing the reaction time, the amplitude of absorption peak also increases. The maximum of absorption wavelength of metal based nanoparticles depends on different factors such as particle size, shape and distribution. It also dependences on the dielectric constant of medium. In this case, absorption peaks indicate the formation of Ag nanoparticles in spherical shape with 7–20nm of average diameter [32]. In (Figure 3) shown the UV-vis spectra of Ag nanoparticles synthesized by the root extracts of Scutellaria baicalensis which was prepared into the first variant methods (after 24 hours - 1, after 15 days -2, after 1 month -3) and by the extract which was prepared into the second variant methods (after 24 hours - 4, after 15 days -5, after 1 month -6). The synthesized silver nanoparticles were analyzed for UV – visible spectroscopic studies after the time duration of 24 hours, 15 days and 1 month. Based on the absorption spectra, the biosynthesized Ag nanoparticles by root extract showed surface plasmon resonance bands at about 414nm–461nm interval as shown in (Figure 3). It is identified that the absorption peaks and intensity are change dependence on the exposition and methods of extract preparation. Since was obtained that during increase exposition time the peak of absorption shifted from 447nm to 461nm in first variant of extract. It shows that in this case the size of Ag nanoparticles increases. This trend is also observed when the Ag nanoparticles were synthesized by the second variant of extract preparation as the peak of absorption shifted from 414nm to 456nm.
FTIR analysis
It is suggested that the biological molecules in plant extract is playing the dual role in the synthesis of metal nanoparticles. They are as a reducing and stabilizing or capping agent. These ability of plant extracts is recognized by comparing the FT-IR spectra of pure plant extract and extract mediated with Ag nanoparticles. FTIR analysis is one of appropriate method for the identification possible functional groups of biomolecules which performs the stabilization factors in the formation of AgNPs (Figure 4). showed the FT-IR spectra of pure powder of root .
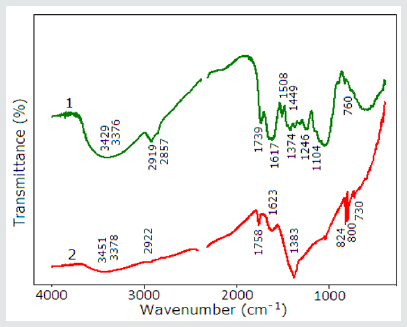
Figure 4: The FT-IR spectra of pure powder of root (1) and root extract mediated with Ag nanoparticles (2).
1. And root extract mediated with Ag nanoparticles
2. The FT-IR spectra of the root powder showed broad peaks at around 3429cm-13) ( N-H stretching vibration), at 3376cm-1 (N-H stretching vibration), 2919cm-1 (O-H stretching of water, flavonoids and carbohydrates), 2857cm-1 (O-H stretching vibration), 1739cm- 1 (C=O stretching vibration), 1617cm-1 1 (strong ring stretching vibration, Amide II (N-H deformation and C-N stretching vibration), 1508cm-1 (strong ring stretching vibration, strong Amide II, medium C=C stretching vibration), 1449cm-1 (-CH2, scissoring vibration) , 1374cm-1 (strong C-O stretching vibration), 1246cm-1 (strong C-N stretching vibration), 1104cm-1 (strong O-C stretching vibration), 760cm-1 (medium, weak C-H deformation vibration).
But the FT-IR spectra of root extract mediated with Ag nanoparticles showed peaks at around 3451cm-1 (medium N-H stretching vibration), 3378cm-1 (weak N-H stretching vibration), 2922cm-1 (medium NH stretching vibration), 1758cm-1 (medium C=C stretching vibration), 1623cm-1 (strong –medium ring stretching vibration, medium-weak, Amide II (N-H deformation and C-N stretching vibration), 1383cm-1 (s-m) (strong - medium C-O string vibration), 824cm-1 (strong CH2 out-of-plane deformation vibration, 800cm-1 (weak - medium; C-H out-of-plane deformation vibration), 730cm-1 (weak-medium C-C skeleton vibration -rocking). The shifts, corresponding to amine, amide and C-O functional groups, show the coordination between zero-valent silver and above-mentioned groups. Absence of peek, corresponding to carbonyl group, shows that reduction goes through these groups. According to the data of other authors for the C–N stretch vibrations corresponds to peaks at 1382cm-1, as well as to the amide I bands of proteins [33]. The peak at 1074cm-1 corresponds to the linkages and shows the presence of flavanones on the surface of nanoparticles [34]. The peaks 1315–1037cm-1 and 1456–1600cm-1 regions corresponds to the phenolic groups of the plant extract [35]. For the C=C stretching in the aromatic ring corresponds to the peak 1592cm-1 and confirm the presence of the aromatic group [36]. They we can assume that flavonoids in the extract of Scutellaria baicalensis might be actively involved and responsible for the reduction of Ag+ to Ag0 [37].
Scanning electronic microscope
(Figure 5) shows typical results the studies of root powder of Scutellaria baicalensis containing Ag nanoparticles deposited on a Al substrate by means of SEM. Part (a) of the figure represents the view of the sample at 80 000× magnification which stands for examining area of about 1.2mkm surface. İn the examined area, one can notice the presence of nanoparticles of sizes within 16.19nm and 18.8nm. Parts (b,c,d) of the figure represents the view of the sample at 220 000× , 330 000× , 500 000× magnification respectively. SEM images showed that most of the silver nanoparticles are predominately spherical in shape having smooth surface and well dispersed with close compact arrangement. The smallest size of nanoparticle was found around 7.12nm. The SEM images shows that nanoparticles may direct contact even within the aggregates, indicating that stabilizater agents act [38]. During the aggregations the small nanoparticles become larger. In (Figure 6) are shown the SEM pictures of synthesized Ag nanoparticles. Usually Ag nanoparticles show at 3 keV a typically strong signal peak , due to surface plasmon resonance [39,40]. In the (Figure 6). are shown the results of elements analysis of the sample which contains Ag nanoparticles. It is clear from fig.6 that elements such as Ag, O, C, K, Cl, Ca and Na are in the it.
Conclusion
In conclusion, there has been a great increasing interest in green synthesis of Ag nanoparticles. In this study, Ag nanoparticles were synthesized by an ecofriendly and convenient method using the Scutellaria baicalensis root and leaf extracts at ambient temperature. The results of this experiments confirm that root extract of Scutellaria baicalensis may used as a reductant for the synthesis of silver nanoparticles. During biosynthesized of AgNPs are occurs color change of solution, the UV-Vis spectroscopic analysis shows that by absorbtion peak at 414nm–461nm interval nanoparticles quantitatively was monitored. Further characterization with SEM analysis shows that synsized AgNPs have a the spherical form, are polydisperse and size ranging from 7.12 nm to 18.8nm with an average size of 11.35nm.
References
- Klaus T, Joerger R, Olsson E, Granqvist CG (1999) Silver-Based Crystalline Nanoparticles, Microbially Fabricated. J Proc Natl Acad Sci USA 96: 13611-13614.
- Nair B, Pradeep T (2002) Coalescence of Nanoclusters and Formation of Submicron Crystallites Assisted by Lactobacillus Strains. Cryst Growth Des 2: 293-298.
- Konishi Y, Uruga T (2007) Bioreductive Deposition of Platinum Nanoparticles on the Bacterium Shewanella algae. J Biotechnol 128: 648-653.
- Willner I, Baron R, Willner B (2006) Growing Metal Nanoparticles by Enzymes. J Adv Mater 18(9): 1109-1120.
- Vigneshwaran N, Ashtaputre NM, Varadarajan PV, Nachane RP, Paraliker KM, et al. (2007) Biological Synthesis of Silver Nanoparticles Using the Fungus Aspergillus flavus. Mater Lett 61: 1413-1418.
- Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M (2006) Synthesis of Gold and Silver Nanoparticles Using Aloe Vera Plant Extract. J Biotechnol Prog 22: 577-583.
- Jae YS, Beom SK (2009) Rapid Biological Synthesis of Silver Nanoparticles Using Plant Leaf Extracts. Bioprocess Biosyst Eng 32: 79-84.
- Ponarulselvam S, Panneerselvam C, Murugan K, Aarthi N, Kalimuthu K, et al. (2012) Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their antiplasmodial activities. Asian Pacific Journal of Tropical Biomedicine, 574-580.
- Shiv Shankar S, Rai A, Ahmad A, Sastry M (2004) Rapid synthesis of Au, Ag and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Inter Sci 275: 496-502.
- Pramanik, Nilkamal, Aditi Bhattacharyya, Patit Paban Kundu (2015) Spectroscopic analysis and catalytic application of biopolymer capped silver nanoparticle, an effective antimicrobial agent. Journal of Applied Polymer Science 132: 41495.
- Otamiri M, Nilsson KGI (1999) Analysis of human serum antibody– carbohydrate interaction using biosensor based on surface plasmon resonance. International Journal of Biological Macromolecules 26: 263- 268.
- Ip M, Lui SL, Poon VKM, Lung I, Burd A (2006) Antimicrobial activities of silver dressings: an in vitro comparison. J Med Microbiol 55: 59-63.
- Xu X, Brownlow WJ, Kyriacou SV, Wan Q, Viola JJ (2004) Real-Time Probing of Membrane Transport in Living Microbial Cells Using Single Nanoparticle Optics and Living Cell Imaging. Biochemistry 43:10400- 10413.
- Huo S, Xue X, Li Q, Xu S, Cai W (2006) Extending in Situ Attenuated-Total- Reflection Surface-Enhanced Infrared Absorption Spectroscopy to Ni Electrodes. Journal of Physical Chemistry B 110: 25721-25728.
- Hua L, Chen J, Ge L, Ta SN (2007) Silver nanoparticles as matrix for laser desorption/ionization mass spectrometry of peptides. Journal of Nanoparticle Research 9(6): 1133-1138.
- Wiley BJ, Chen Y, McLellan JM, Xiong Y, Li Z, et al. (2007) Synthesis and optical properties of silver nanobars and nanorice. Nano Letters 7: 1032-1036.
- Dubas ST, Pimpan V (2008) Green synthesis of silver nanoparticles for ammonia sensing. Talanta 76: 29-33.
- Mishra YK, Mohapatra S, Kabiraj D, Mohanta B, Lalla NP, et al. (2007) Synthesis and characterization of Ag nanoparticles in silica matrix by atom beam sputtering. Scripta Materialia 56: 629- 632.
- Xiong D, Chen M, Li H (2008) Synthesis of para-sulfonatocalix[4]arenemodified silver nanoparticles as colorimetric histidine probes. Chemical Communications 7: 880- 882.
- Zhejiang J, Yuan CY, Anhui L, Hailing T, Mingle T, et al. (2007) Science in China Series B 50: 345-350.
- Dubas ST, Pimpan V (2008) Humic acid assisted synthesis of silver nanoparticles and its application to herbicide detection. Materials Letters 62: 2661-2663.
- Bonsak J, Mayandi J, Thøgersen A, Marstein ES, Mahalingam U (2011) Chemical synthesis of silver nanoparticles for solar cell applications. Phys Status Solidi C 8: 924–927.
- Nanoparticles Inspire Plasmonic Solar Cell. Technical Articles & Reports on Plastic Industry: Silver Nanoparticles Can Increase Electrical Power Generation of Polymer Solar Cells.
- McFarland AD, Van Duyne RP (2003). Single silver nanoparticles as realtime optical sensors with zeptomole sensitivity. Nano Lett 3: 1057–1062.
- Lukman AI, Gong B, Marjo CE, Roessner U, Harris AT (2011) Facile synthesis, stabilization, and anti-bacterial performance of discrete Ag nanoparticles using Medicago sativa seed exudates. J Colloid Interface Sci 353: 433–444.
- Rodríguez-León E, Iñiguez-Palomares R, Navarro RE, Herrera-Urbina R, Tánoris J, et al. (2013). Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res Lett 8: 318.
- Skinner HCW, Jahren AH (2003) Biomineralization. In: Schlesinger WH (Edt.), Treatise on Geochemistry, Elsevier, Amsterdam, Netherlands, pp. 117–184.
- Joshee N, Patrick TS, Mentreddy RS, Yadav AK (2002) Skullcap: Potential medicinal crop. pp. 580–586. In: Janick J, Whipkey K (Eds.), Trends in new crops and new uses. ASHS Press, Alexandria, VA. Hardback, USA, p. 599.
- Janick J, Whipkey A (2006) Trends in new crops and new uses. ASHS Press, Alexandria, VA. Hardback: p. 599.
- Heo HJ, Kim DO, Choi SJ, Shin DH, Lee CY (2004) Potent inhibitory effect of flavonoids in Scutellaria baicalensis on amyloid beta protein-induced neurotoxicity. J Agr Food Chem 52: 4128–4132.
- Hwang JM, Tseng TH, Tsai YY, Lee HJ, Chou FP, et al. (2005) Protective effects of baicalein on tert-butyl hydroperoxide-induced hepatic toxicity in rat hepatocytes. J Biomed Sci 12: 389–397.
- Klaus B Mogensen, KatrinKneipp (2014) Size-Dependent Shifts of Plasmon Resonance in Silver Nanoparticle Films Using Controlled Dissolution: Monitoring the Onset of Surface Screening Effects. J Phys Chem C 118: 28075-28083.
- Gurunathan S, Jeong JK, Han JW, Zhang XF, Park JH, et al. (2015) Multidimensional effects of biologically synthesized silver nanoparticles in Helicobacter pylori, Helicobacter felis, and human lung (L132) and lung carcinoma A549 cells. Nanoscale Res Lett 10: 35.
- Shankar SS, Rai A, Absar Ahmad A, Sastry M (2004) Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using neem (Azadirachtaindica) leaf broth. J Coll Interface Sci 275: 496–502.
- Jeeva K, Thiyagarajan M, Elangovan V, Geetha N, Venkatachalam P (2014) Caesalpiniacoriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Ind Crops Prod 52: 714–720.
- Reddy NJ, Vali DN, Rani M, Rani SS (2014) Evaluation of antioxidant,antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater SciEng C 34: 115–122.
- Zuas O, Hamim N, Sampora Y (2014) Bio-synthesis of silver nanoparticles using water extract of Myrmecodiapendans (Sarang Semut plant). Mater Lett 123: 156–159.
- Priya AM, Selvan RK, Senthilkumar B, Satheeshkumar MK, Sanjeeviraja C (2011) Synthesis and characterization of CdWO4 nanocrystals. Ceramics International 37(7): 2485-2488.
- Kaviya S, Santhanalaksnmi J, Viswanathan B, Muthumany J, Srinivasan K (2011) Biosynthesis of silver nanoparticles using citrus sinensis peel extract and its antibacterial activity. Spectrochim Acta Part A 79: 594– 598.
- Das J, Das MP, Velusamy P (2013) Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochim Acta Part A 104: 265–270.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...