Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-6660
Research Article(ISSN: 2637-6660) 
Development and Characterization of Glandless Cottonseed Meal/Pullulan Fine Fiber Mats Volume 1 - Issue 4
Loreana Molero1, Luissanyi Campos1, Luis Sosa1, Yuanbing Mao2, Nancy Flores3, Efren Delgado4 and Karen Lozano1*
- 1 Mechanical Engineering Department, The University of Texas Rio Grande Valley, USA
- 2Chemistry Department, The University of Texas Rio Grande Valley, USA
- 3Extension Family Consumer Science, New Mexico State University, Mexico
- 4Family Consumer Science, New Mexico State University, Mexico
Received: June 27, 2018; Published: July 03, 2018
*Corresponding author: Karen Lozano, Mechanical Engineering Department, The University of Texas Rio Grande Valley, Edinburg TX 78539, USA, Email: karen.lozano@utrgv.edu
DOI: 10.32474/ANOAJ.2018.01.000117
Abstract
Glandless cotton seed meal (GCSM) extracted from cotton is composed of nearly 50 percent protein. For about every 100kg of cotton fiber produced the cotton plant yields approximately 160kg of cottonseed. GCSM presents a unique opportunity to be used in the food industry. In this study, GCSM based polymer composite edible fine fibers were successfully produced using pullulan as the polymeric base. GCSM solutions were developed and spun as fine fibers using a centrifugal spinning technology. A synergistic approach among fiber output and fiber diameter was pursued by modifying angular velocity and concentration of pullulan and GCSM within an aqueous solution. The developed fibers show average fiber diameters of 720nm with a standard deviation of 200nm. Thermo-physical analysis was conducted. The opportunity to develop GCSM fine fiber mats opens up potential applications in food packaging and drug delivery systems to mention some.
Keywords: Nanofibers, Pullulan, Glandless Cotton Seed Meal, Forcespinning®
Introduction
In the past few years, fine fibers (nano and submicron) have attracted significant scientific interest mostly because of their high surface area to volume ratio and porous structure within and among the developed nanofibers. These properties have given rise to numerous promising applications such as filtration, smart textiles, sensors, solar cells, fuel cells, batteries, etc. In the biomedical area, nano fiber mats have received increasing attention due to potential to solve a variety of existing challenges in burn and wound care, tissue regeneration and treatment for a variety of diseases given its ability to enhance adhesion of drugs, proteins, and cells [1]. The focus of published nano fiber studies pertains to the development and production of nanofibers and investigation of their chemical, electrical, thermo-physical, rheological, and mechanical properties, correlated to the applications mentioned above. Few studies have related the use of nanofibers in food science and engineering fields. There are a variety of applications to explore ranging from food preservation and packaging to more complex ones such as nutrient delivery systems. Recently, the Forcespinning® technology has garnered significant attention for nano, submicron, and single digit microfiber development. The reason behind this tendency is due to its unprecedented yield when compared to other methods such as electro spinning and wet chemistry methods. Forcespinning® is a novel fine fiber production method where the electrical forces used in electro spinning are replaced by centrifugal forces. The Forcespinning® technique provides ample flexibility in materials selection since electric fields are not needed; therefore no need to select solvents with appropriate dielectric parameters. Water based systems can be effectively utilized as well as melt spinning, further lowering production costs by removing the need to budget solvent recovery steps. In Forcespinning®, centrifugal force is used to extrude the solution/melt through small orifices with a controlled length to diameter ratio [2]. As the extruded fibers exit the rotating spinneret; air drag forces elongation of the fibers and reduces their diameter to the nanometer scale. At the same time that fibers are being stretched the solvent evaporates (or melt cools down) leaving solidified fibers. The fibers can then be collected by different methods allowing the creation of non-woven mats, aligned fiber mats, or yarns. The system has spinnerets specifically designed either for lab scale or industrial scale systems. Spinnerets used in lab scale systems have 2 to 6 nozzles and make use of needles to effectively promote the needed aerodynamic environment that promotes the formation of homogenous, long continuous fine fibers. At the industrial scale, there are hundreds of nozzles per spinneret and no needles are used. Polymeric, ceramic, metal oxides and composite fine fiber mats have been prepared by this method, for example, polyvinyl di fluoride [3], polypropylene [4], chitosan-based composites [5], vanadium pentoxide [6], titanium dioxide [7], and indium tin oxide fibers [8] to mention some.
Currently, there is a wide variety of biopolymers used in the food industry. These biopolymers possess attractive properties such as biocompatibility, biodegradability and most importantly, are non-toxic. Pullulan (PLL), a non-ionic exopolysaccharide that is obtained from the starch of the fungus Aureobasidium pullulanshas recently received attention in the food industry due to its distinctive characteristics that include water-solubility, high water absorbing capacity, and capability to form strong, odorless, tasteless, and flexible films and fibers Kristo et al. [9]. Studies have shown that pullulan has potential for mass production of fibers [5]. In addition to being an effective oxygen barrier pullulan has been utilized in the food industry for thickening, gelling, and as a stabilizing agent [10]. It is also used to extend shelf life, control the respiration rate, and to prevent the growth of microorganisms on fruits and vegetables [11]. Glandless Cottonseed Meal (GCSM) extracted from cotton, presents a unique opportunity to be used in the food industry. Cotton is not only a major fibrous crop, but also responsible for second largest oil and protein production in the world, second to soybeans [12]. The cotton plant produces 160kg of cotton seed while yielding 100kg of fiber [13]. Processing the cottonseed results in byproducts such as oil, hulls, linters, and meal/flour [14]. Cottonseed meal is the remaining product after cotton fibers are separated from their seeds.
Cottonseed, often fed as livestock feed or used as fertilizer, has a nutrient content of 6 to 7 percent nitrogen, 2 to 3 percent P2O5, and 1 to 2 percent K2O [15]. GCSM contains 43 percent protein, 15.5 percent fiber and 0.38 percent of gossypol [16]. Gossypol is a toxic natural compound found in cottonseeds, its main purpose is to protect the plant from insects. Elimination of gossypol allows use of the glandless meal for animal feed, organic fertilizers, and other food related applications. GCSM can be used as meal replacement because it is high in protein and less expensive than fishmeal and soybean meal [17]. GCSM has shown promising results in the incorporation of protein to cereal and flour based extruded snacks [18]. This study focuses on the development of edible nanofibers using GCSM protein with pullulan as the polymer precursor. To the best of the authors’ knowledge, this is the first study conducted on the development of nano fiber based membranes containing GCSM protein. Edible nanofibers have potential applications in food packaging, as nutritional additives and in drug delivery to mention some.
Materials and Methods
Materials
Pullulan (PULL) powder was purchased from Tokyo Chemical Industry Co., LTD. Glandless cottonseed meal (GCSM) was provided by Cotton Inc. (Texas, USA) containing 48.5 percent of crude protein and 11.8 percent of lipids. It was stored at 4°C until used. Protein was extracted utilizing a 0.1 M KOH solution at a 1:12 ratio (w/v; pH 12) via a thermal process at 65°C for 40 minutes. The liquid was centrifuged at 3500 rpm for 20min (RCF 4793g) at 4°C. Recovered supernatant was acidified to pH 4.5 with 0.5MHCl and distilled water was used to precipitate the protein. It was then centrifuged at 3500rpm for 20min at 4°C to recover protein solids. Protein solids were re-suspended in distilled water for storage and transportation in between laboratories [19]. Figure 1 shows the several steps used to prepare the fibers. The process started with developing pullulan solutions. Pullulan in its powder form was mixed with distilled water; 2.13g of PULL were used to prepare a 21 wt percentage PULL solution. The samples were prepared at room temperature and magnetically stirred for 2 hours. GCSM protein powder was obtained through vacuum filtration and added to the PULL solutions, two samples were prepared containing 5 and 8 wt. percentage of GCSM. The PULL solutions with the added GCSM were magnetically stirred in an oil bath for 2 hours at room temperature.
Experimental
PULL and PULL/GCSM nanofibers were produced by the Forcespinning® technique, using a Cyclone L-1000M (manufactured by Fiberio Technology, Corp. USA). The spinneret equipped with 30G half-inch needles (Becton Dickinson and Company) at each end was filled with 1 mL of the developed solutions. The solutions were spun at different RPM (4500-8500) at room temperature under 52±3 R.H. The system was allowed to spin for three minutes. Nonwoven fiber mats were collected in between pillars (eight pillar collector system shown in Figure 1). Samples were placed in plastic bags for future characterization and desiccant was added to prevent moisture absorption. Morphological characterization of the PULL/GCSM nanofibers was conducted utilizing a Scanning Electron Microscope (SEM Carl Zeiss, model Sigma VP, Germany). The average fiber diameter was calculated using the Image J version 1.48 software, 100 fibers were measured per sample taken from at least 20 micrographs and conducting 3 measurements per fiber, 300 values were used. Thermal behavior of PULL and PULL/ GCSM fibers was studied using a Differential Scanning Calorimeter (DSC, model Q-100 TA Instruments, USA) under air and nitrogen flow conditions, the samples (5mg) were ramped from room temperature to 280°C, isothermal for 10 minutes, and then ramped down to 30°C. This cycle was repeated to account for thermal history effects. The scanning rate was 5°C/min. Thermogravimetric Analysis (TGA, model Q-500 TA Instruments, USA) was conducted using platinum crucibles. Samples of approximately 10 mg were tested under nitrogen purge, with a flow rate of 40ml/min. The samples were ramped from room temperature to 800°C at a heating rate of 10°C/min.
Figure 1: A solution of GCSM and pullulan was prepared and further used to develop fine fibers using the Forcespinning® process. From top right corner, clockwise. The process begins with the GCSM aqueous solution which, is placed under vacuum filtration to remove water. The GCSM and pullulan powders are then weighted, and desired concentrations placed in an aqueous solution. This solution is then spun using the Cyclone L-1000M which uses a two-nozzle spinneret. The developed fibers are then collected in 4”x4” mats.

Results and Discussion
Fiber development was conducted at angular velocities starting from 4500 RPM up to 10000 RPM. It was observed that successful fiber production started at velocities higher than 5000 RPM, specifically at 5500 RPM and up to 8000RPM. These range was considered the optimum for the pure pullulan, 5 and 8 weight percent GCSM solutions, respectively. Fibers were produced for all systems within the angular velocity range of 4500 to 8500RPM though to determine optimum angular velocity, a synergy between fiber output and fiber diameter was considered. For example, fibers with diameters of less than 100nm were observed though the fiber output was not significant. It was observed that the optimal RPM was around 5000-6000 RPM, the yield fiber output was the highest. Surpassing the previously stated speeds, the fiber output would significantly drop. At lower (< 5000) the fiber output was severely impacted; the fiber production was low, with beady morphology. Figure 2 shows scanning electron micrographs of the developed fiber mats with its corresponding fiber diameter statistical analysis. Figure 2a shows results for pure pullulan fibers, 2b for fibers prepared with 5weight percent of GCSM, and 2c for fibers with 8 weight percent of GCSM. As observed, homogeneous, long, and continuous fine fibers were developed. Bead formation was not observed. The addition of the GCSM resulted in a small increase in fiber diameter being statistically insignificant for the different concentrations. The average fiber diameters were 720nm respectively with a standard deviation of 200nm. The developed nonwoven fiber mats are as shown in Figure 1, homogeneous, flexible and easy to handle.
Figure 2: (A) Pure pullulan fibers spun at 5500 rpm, (B) composite fibers containing 5wt percentage of GCSM spun at 7000rpm, (C) composite fibers containing 8 % wt. of GCSM spun at 8000rpm, and (D) statistical analysis of fiber diameter.
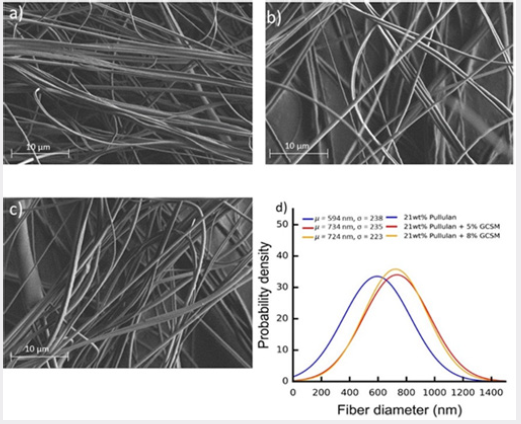
Figure 3: Dynamic scanning calorimetry thermo grams for the pullulan in bulk state (powder), and fiber form for pullulan and its GCSM composites. Figures 3a and 3b, show first heating and first cooling, respectively, of the analyzed fibers.
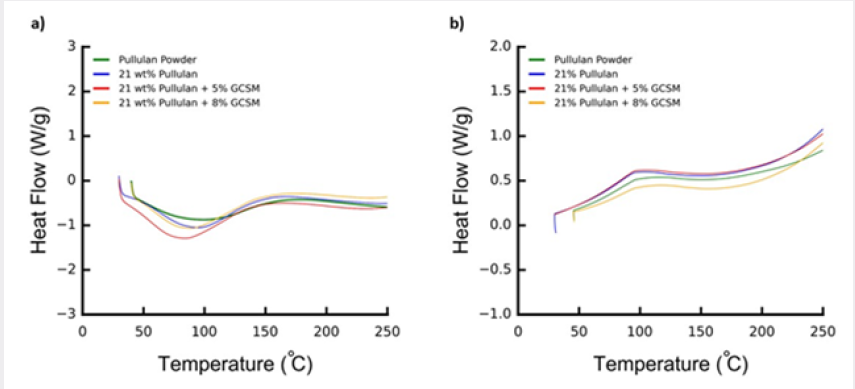
DSC analysis was performed in order to assess the effect of molecular elongation on the microstructure of the polymer. As can be seen in Figure 3, the DSC thermographs of the pullulan in bulk state and fiber state differ significantly. Pullulan powder is an amorphous polymer. As depicted in Figure 3, there was no melting transition, but subtle endothermic (Figure 3a) and exothermic (Figure 3b) transitions were observed attributed to the glass transition temperature (Tg). Upon spinning the polymer into fiber form, a clear change in the location and magnitude of the glass transition temperature is observed. First of all, the location of the Tg is shifted to lower temperature implying a plasticizing effect upon fiber formation, alignment of polymer molecules. Also, for the nonwoven fiber mats, the Tg depicted as a step in the bulk pullulan sample is now seen as an endothermic Tg peak. There is no evidence of cold crystallization (considering the great chain flexibility of pullulan) though, fiber formation certainly affected chain mobility and was manifested in the development of the observed Tg peak attributed to enthalpy relaxation or enthalpy recovery. This endothermic peak (in the fiber sample) allows to quantitatively analyze relaxation kinetics of amorphous systems, often conducted with moisture absorption tests [20]. In this case, the decrease in Tg and presence of these broad transition peaks suggests certain degree of molecular alignment and the tendency of the largely flexible chain to attain equilibrium. The fiber samples mimic a plasticized system. The observed plasticizing effect implies an increase in free volume and segmental movements, where consequently, water molecules can easily diffuse. These results could provide attractive opportunities to the use of pullulan fiber systems and GCSM/pullulan composites in edible systems and drug delivery applications. It is to be noted that the exothermic transitions presented in Figure 3b occur all at the same temperature, the thermal history of the polymer due to fiber processing has been erased and now the bulk systems present a transition similar to the one obtained for the raw pullulan.
Figure 4: Weight loss as a function of temperature for the GCSM, pullulan in powder and fiber form and the two (5 and 8 wt percentage) composite fiber mats.
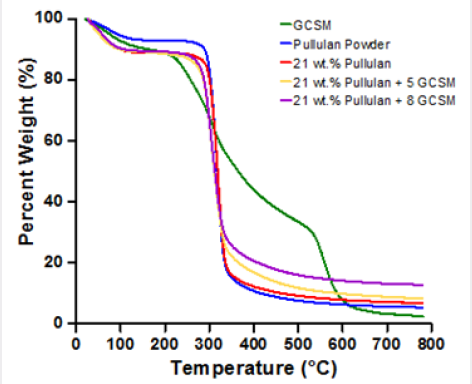
Thermo gravimetric analysis of the developed systems is shown in Figure 4. In this graph, it is clear that fiber samples absorb more humidity compared to the pullulan in powder form as can be seen in the weight loss occurring at around 100ºC. The hydrophilicity of the polymer coupled with increased surface area due to fiber formation promotes a higher absorption of humidity, about 10 weight percent for all fiber samples. The fiber samples containing GCSM show a small reduction in thermal stability when compared to the pure pullulan fibers, this driven by the initial weight loss of the GCSM protein occurring at 211˚C and attributed to rapid decomposition of small molecules (glycerol and protein domains) [20,21]. The main degradation occurs at 342°C and 347°C for the pullulan powder and pullulan fibers, respectively; and at 343°C for 5 weight percent and 340°C for 8 weight percent GCSM composite fibers.
Conclusion
Developing of nano and submicron fibers containing GCSM protein opens an opportunity to better utilize this abundant source of protein. Fine fibers containing pullulan as the polymer precursor reinforced with GCSM protein were developed. The developed pullulan and pullulan/GCSM solutions showed ease of fiber processing at angular velocities in between 5500 and 8000RPM. The collected fibers were homogenous, long and continuous, with average diameters in the range of 650nm. Fine fiber diameters such as less than 100nm were also obtained though not is sufficiently high yield as to promote practical applications. The PLL/GCSM composite fibers showed a decrease in Tg when compared to the pullulan in its powder form. This decrease is attributed to induced alignment of the pullulan molecules. Further studies will focus on the viability of using these fibers as products with high nutritional protein content for human consumption and other potential applications such as in drug delivery and novel snacks to mention some.
References
- Leung V, Frank K (2011) Biomedical Applications of Nanofibers. Polymers for Advanced Technologies 22(3): 350-365.
- Sarkar K, Gomez C, Zambrano S, Ramirez M, De Hoyos E, et al. (2010) Electro spinning to Forcespinning. Materials Today 13(1): 12-14.
- Vasquez B, Vasquez H, Lozano K (2012) Preparation and Characterization of Polyvinylidene Fluoride Nanofibrous Membranes by Forcespinning. Polymer Engineering & Science 52(10): 2260-2265.
- Raghaven B, Soto H, Lozano K (2013) Fabrication of Melt Spun Polypropylene Nanofibers by ForcespinningTM. Journal of Engineered Fibers and Fabrics 8(1): 52.
- Xu, F, Weng B, Materon LA, Gilkerson R, Lozano K (2015) Development of Tannic acid/chitosan/pullulan Composite Nanofibers from Aqueous Solution for Potential Applications as Wound Dressing. Carbohydrate Polymers 115: 16-24.
- Altecor A, Li Q, Lozano K, Mao Y (2014) Mixed Valent VO Sub.x /polymer Nanohybrid Fibers for Flexible Energy Storage Materials. Ceramics International 40(3): 5073.
- Gutierrez H, Villarreal G, Lozano K, Vasquez H (2015) Titanium Dioxide Nano fibers through Force spinning. Journal of Engineered Fibers and Fabric 2: 129-136
- Altecor A, Mao Y, Lozano K (2012) Large-Scale Water Based Production of Tin-Doped Indium Oxide Nanofibers by Forcespinning®. Functional Materials Letters 5(3): 1250020.
- Kristo E, Biliaderis CG, Zampraka A (2007) Water Vapour Barrier and Tensile Properties of Composite Caseinate-Pullulan Films: Biopolymer Composition Effects and Impact of Beeswax Lamination. Food Chemistry 101(2): 753-764.
- Farris S, Unalan IU, Introzzi L Fuentes Alventosa JM, Cozzolino CA (2014) Pullulan‐based Films and Coatings for Food Packaging: Present Applications, Emerging Opportunities, and Future Challenges. Journal of Applied Polymer Science 131(13): 40539.
- Falguera V, Quintero JP, Jiménez A, Muñoz JA, Ibarz A (2011) Edible Films and Coatings: Structures, Active Functions and Trends in their use. Trends in Food Science & Technology 22(6): 292-303.
- Lusas EW, Jividen GM (1987b) Glandless cottonseed: a review of the first 25 years of processing and utilization research. Journal American Oil Chemistry Society 64(6): 839-854.
- Li JT, Li DF,Zang JJ, Yang WJ, Zhang WJ, Zhang LY (2012) Evaluation of energy digestibility and prediction of digestible and metabolizable energy from chemical composition of different cottonseed meal sources fed to growing pigs. Asian Australasian Journal of Animal Sciences 25(10): 1430-1438.
- Tanksley TD (1990) Nontraditional Feed Sources for Use in Swine Production (10th edn.); Department of Animal and Poultry Science, Butterworth Publishers, Elsevier.
- Blasi DA, Drouilland J (2002) Composition and feeding value of cotton seed feed products for beef cattle. Kansas State University, USA.
- La Rue DC, Knabe DA, Tanksley TD (1985) Commercially Processed Glandless Cottonseed Meal for Starter, Grower and Finisher Swine. Journal of Animal Science 60(2): 495-502.
- Heywang BW (1947) A Comparison of Cottonseed and Soybean Meal in the Diets of Laying Chickens. Poultry Science 26: 442
- Reyes-Jáquez D, Casillas F, Flores N, Andrade-González I, Solís-Soto A, et al. (2012) The effect of glandless cottonseed meal content and process parameters on the functional properties of snacks during extrusion cooking. Food and Nutrition Sciences 3(12): 1716-1725.
- Valverde-Quiroz LM (2017) Protein Extraction from Glandless Cottonseed Meal as a Functional Food Ingredient. Master of Science Thesis, New Mexico State University, Las Cruces NM, USA.
- Biliaderis CG, Lazaridou A, Arvanitoyannis I (1999) Glass Transition and Physical Properties of Polyol-Plasticised pullulan-starch blends at Low Moisture. Carbohydrate Polymers 40(1): 29-47.
- Yue H, Yin G, Cui Y (2016) Glandless Cottonseed Protein for Environmentally Friendly Bio plastics. Cotton Research, Ibrokhim Abdurakhmonov.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...