Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2690-5787
Research Article(ISSN: 2637-4692) 
Efficacy Evaluation of a Lytic Bacteriophage Cocktail against Carbapenem – Resistant Pseudomonas Aeruginosa in a Wound-Infection Model of Swiss Mice Volume 1 - Issue 3
Ivan Ibanda1*, Jesca Nakavuma2 and Claude Kirimuhuzya1,3
- 1Department of Pharmacology and Toxicology, School of Pharmacy, Kampala International University, Bushenyi, Uganda
- 2Department of Biomolecular and Biolab Sciences, College of Veterinary Medicine, Animal Resources and Biosecurity, Makerere University, Kampala, Uganda
- 3Department of Pharmacology, School of Medicine, Kabale University, Kabale, Uganda
Received:September 11, 2023; Published:September 21, 2023
Corresponding author: Ivan Ibanda, Department of Pharmacology and Toxicology, School of Pharmacy, Kampala International University, Bushenyi, Uganda
Abstract
Introduction: Bacteriophages can easily be isolated from environments such as hospital wastewater and enriched with targeted bacteria. Phage products can also be developed faster and more cost-effectively than conventional drugs. However, there is a lack of infrastructure and limited experience in phage therapy in LMICs amidst a heightened level of antimicrobial resistance and their application has not been fully adopted in Uganda. This study was to evaluate the efficacy of a locally formulated lytic bacteriophage cocktail against carbapenem-resistant Pseudomonas aeruginosa (CRPA) clinical isolates in a wound-infection mouse model.
Methods: The study involved the evaluation of the in vivo efficacy of a 3-bacteriophage cocktail on lysing CRPA in wounds of 25 Swiss male mice with body weights of 20 to 30 g. Two wounds were created on the upper back of each mouse after which they were contaminated with CRPA. Wound closure was monitored and measured for 10 days.
Results: The mean wound diameters at the end of the experiment showed that the phage cocktail-treated wounds registered significant closures of at least 67.7% and these measurements were statistically different from those of the meropenem-treated wounds. Wound closure was observed to increase over time from day one to the tenth day of the experiment. The locally formulated and optimized three-phage cocktail demonstrated satisfactory efficacy in the treatment of wounds infected with carbapenem – resistant Pseudomonas aeruginosa clinical isolate when topically administered in sufficient doses and at optimal frequencies.
Keywords: Bacteriophage; cocktail; carbapenem resistant; pseudomonas aeruginosa; wound healing; mouse model
Introduction
Bacteriophages (phages) are therapeutic agents that had been used for more than a century in the treatment of bacterial infections but were sidelined after the discovery of antibiotics [1]. The rediscovery and continued implementation and development of bacteriophage therapy by countries like Georgia, Russia, and Poland, might be one solution to the global threat of escalated antibiotic failure as an alternative non-antibiotic approach to conventional antimicrobial therapy [2-4]. This is based on the fact that, over billions of years, phages have co-evolved with their hosts (bacteria), developing mechanisms to counter bacterial defenses such as extracellular biofilm production, which significantly reduces the efficacy of conventional antibiotics [5]. Bacteriophage therapy primarily uses obligately lytic phages to kill their respective bacterial hosts while leaving human cells unharmed and reducing the wider impact on commensal bacteria that antibiotic use frequently has [6].
Lytic bacteriophages are defined as specific, non-toxic, selfreplicating viruses that can eradicate bacteria that are both sensitive and resistant to antibiotics, making them a key drug class that could save many lives threatened by the antimicrobial resistance crisis [7,8]. Because lytic bacteriophages against ESKAPE pathogens can be isolated from hospital wastewater and anywhere the target bacteria are present, they are readily available therapeutic agents [9,10]. There are an estimated 1031 phage particles on this planet, an impossibly large number that equates to approximately a trillion phages for every grain of sand on the planet, with profound effects on a wide range of biological and environmental processes. Moreover, phages also produce enzymes that are active against bacteria, such as endolysin Cpl-1, which is effective against pneumococcal pneumonia [11].
The use of phage therapy for Pseudomonas aeruginosa infections dates from the early 1990s. This occurred during a period in which laboratory studies of local and systemic infections were followed by clinical trials in which symptomatic improvement and phage multiplication were observed in a pet dog with otitis and a human with an infected burn [12]. Due to the increasing prevalence of antibiotic resistance in this particular bacterium, phage therapy to eradicate Pseudomonas aeruginosa infections has been reborn, resulting in the isolation and characterization of many bacteriophages with lytic activity against it [13-16]. To evaluate their activity in humans, tests have been conducted in both in vitro and in animal (in vivo) setups. The efficacy of phage therapy against infectious diseases caused by P. aeruginosa has been shown in simpler experimental animal models and also in those that closely resemble the pathophysiology of diseases in humans [17]. Most of these studies have yielded very promising results for future use [18-22]. As a result, bacteriophages have the potential to treat antibiotic-resistant P. aeruginosa infections and these findings have been published in a variety of clinical cases, case series, and clinical trials [23-25].
Additionally, the isolation of more phages for the potential formulation of cocktails to control P. aeruginosa infections, on the other hand, has been strongly encouraged. The use of a phage cocktail against specific pathogens increases their killing potential and even slows bacterial regrowth. A good number of studies have been conducted to evaluate the effectiveness of phage cocktails, including a 6-phage cocktail that was designed and shown to lyse clinical strains of P. aeruginosa both in planktonic liquid cultures and in biofilm, a 4-phage cocktail that removed the Clostridium difficile pathogen and favorably modified the model gut microbiome [26], and, last but not least, a 3-phage cocktail that was able to lyse E. coli isolates after incubation in a high saline environment [27]. Although phages have been in use as antibacterial agents in many countries, they are largely a new technology, especially on the African continent, Uganda inclusive, where they have received little attention [28].
Consequently, there is a lack of resident data on the activity of bacteriophages in local environments in many lower middleincome countries. With microbiological tools readily available to people in LMICs, including Uganda and Africa at large, phages can be easily isolated from environments such as hospital waste or sewage water and enriched with targeted bacteria [29]. Phage products, in theory, can also be developed faster and more cost-effectively than conventional drugs, and they can be formulated into dry powder preparations that do not require refrigeration [30]. Furthermore, there is a lack of infrastructure and limited experience in phage therapy in LMICs. It has also been proven that phage specificity requires locally sourced strains that will affect bacteria from the same setting, hence the need for the evaluation of locally isolated phages. Thus, this study is one of the few that has contributed to the understanding and/or application of phage therapy.
Materials and Methods
Ethical statement
The ethical approval for the study was given by the Kampala International University – Research Ethics Committee (KIU-2021- 9) under the Uganda National Council for Science and Technology (UNCST). Additionally, during the laboratory analyses of the proposed study, the researcher (s) strictly adhered to the Covid-19 local policies and procedures to control and prevent infections. These included social distancing, use of face coverings including masks and respirators and personal hygiene and sanitation were ensured through hand sanitizing.
Study design
This was an experimental study that involved formulation and evaluation of the in vivo efficacy of a bacteriophage cocktail against carbapenem-resistant Pseudomonas aeruginosa (CRPA) on the healing of wounds infected with CRPA using a mouse model. The CRPA clinical isolate used was obtained from Microbiology Laboratory, College of Health Sciences at Makerere University, Kampala (Uganda) as reported in the study [31]. The carbapenem resistance status of the clinical isolate was confirmed using the disc-diffusion susceptibility method with interpretation based on the Clinical and Laboratory Standards Institute (2020). The comparisons made were between the controls; Group I (meropenem-treated) and Group II (Colistin-treated), and Groups III-V (phage cocktail-treated). The experimental unit was a single animal and measurements were taken for each of the wounds for every mouse.
Sample size
Sample size determination
Twenty-five Swiss male mice that were at least eight weeks old, with body weights of 20 to 30 g were used. The adequate sample size of the mice used was determined using the power analysis in STATAv15.0. Setting the effect size to 0.88, between-group variance to 0.78, and choosing a statistical power of 90% at a significance level of 5% for a two-tailed analysis, a sample size of 25 mice was determined and evaluated to be adequate. This allocated 5 animals per each of the 5 groups. These were sourced from the animal facility of the Pharmacology Laboratory of Kampala International University (Western Campus).
Inclusion and exclusion criteria
Only male mice were used since the reproductive hormones (most especially estrogen) in the adult females have been suggested to compromise wound healing [32]. At the end of the experiment, all the groups had 5 animals for which the measurements had been done.
Randomization
Randomization was used to allocate animals to the 5 experimental groups created. Each of the 20 animals was labeled with a serial number. The numbers were written down on pieces of paper, placed into a container and properly mixed. The papers were then picked out of the container to select the sample mice for each group at a time. There was no control over confounding factors at the design stage.
Experimental animals
Housing and husbandry
The mice were kept in standard and monitored (using a hygrometer/temperature instrument) conditions of 21±5 °C, relative ambient humidity at the level of mouse cages of 55% +/- 15% (40 - 70), and a 12 h light-dark cycle with free access to food and water ad libitum at a level that allowed them to sit while eating and drinking. To reduce the risk of post-purchase contamination, the feeds were stored in a clean, dry, vermin-free, well-ventilated area. The animals’ living conditions were kept appropriate for their species and contributed to their health and comfort, with cage height not less than 5 inches and a floor area of about 6x15 inches per mouse to allow the mice to stand on their hind legs, stretch up fully, and climb on the cage lid’s bars. The cages were made of non-toxic, non-absorbable materials that were easy to clean and could be made transparent or colorless. They were also longlasting, resistant to heat and chemicals, and predator- and escapeproof. The cages’ floors were solid rather than wire-mesh. Uniform bedding was made available in sufficient quantities to cover the entire floor to a depth of at least 2 centimeters. The material used was hardwood.
Animal care and monitoring
To identify or label the fur and tail, non-toxic dyes and permanent markers were used. To extend the life of the marker identification, these were replaced whenever necessary by swabbing the tail with 70% isopropyl alcohol before marking. All cages were labeled with information about each treatment group. Although wounding the mice causes more than momentary or slight pain or distress to the animals but this was done under anesthesia induced with ketamine to avoid or minimize discomfort, distress, and pain to the animal. The animals were individually caged after wounding to prevent bites from the other mice in the same cage. No animal was used in more than one major operative procedure from which it was allowed to recover. At the end of the study, the animals were painlessly euthanized to relieve them from experiencing what otherwise could have caused severe or chronic pain or distress. Because the surgical techniques required for this model are not overly complex, this model can be widely used by those with little surgical experience. The euthanized animals were incinerated at the end of the study to ensure that the environment is safe from the resistant microorganisms used.
Outcome measure
The percentage wound closure was used to quantify the effect of healing due to the lysis of the CRPA in the mice.
Statistical Methods
The quantitative data [dataset] were entered into Microsoft Excel before being imported into STATAv15.0 for statistical analysis. The average wound diameters were checked for normality using Shapiro – Wilk test. The non-normally distributed data was log transformed. The group differences in mean reductions in wound diameters were analyzed using one-way ANOVA (analysis of variance) at a confidence level of 95%, with a p value less than 0.05 considered statistically significant. To adjust for the p values of the significant differences, a post hoc test (Bonferroni) was used to compare the means of the negative control and test groups. Wound closure (healing) was expressed as a percentage of initial wound diameter at day 1 (baseline) as compared to the diameters for the later daily average measurements. The results were presented as Mean ± SEM and/or percentages in Tables.
Experimental Procedures
Formulation of the bacteriophage cocktail
A 3-phage cocktail, C12, was formulated using newly isolated and purified bacteriophages; P01, P03 and P04, in the ratio of 1:1:2, respectively. These bacteriophages were among the four phages (Pa01 through Pa04) isolated with unique plaque morphological appearances on carbapenem-resistant Pseudomonas aeruginosa from hospital effluents in western Uganda. The phages were isolated according to the method described by [33], with modifications. The purification of the phage isolates was done according to the modified procedure of [34]. Briefly, a 10-fold serial dilution of enriched phage lysate obtained from Section 3.5.1 was conducted using filtered TSB as the diluent. One hundred microliters (100 μl) of an overnight bacterial culture were mixed with 1.5 ml of overlay agar and mixed by inversion. The mixture was then poured onto the appropriately labeled TSA plate and allowed to be set. From the 10- fold diluted phage lysate, 10 μl were spotted on the lawns of the host Pseudomonas aeruginosa. The plates were incubated aerobically at 37°C overnight. Each plate was visually examined and those that contained individual and distinct plaques were selected. The plaques for purification were selected based on plaque morphology (size and clarity). Using a sterile 1ml –pipette tip, selected plaques were scraped out and transferred to 1 ml TSB in Eppendorf tubes and mixed by inversion.
The tubes were incubated at 4°C for 1 hour, then spanned at 15,000 g for 10 minutes before being filtered and spotted on bacterial overlay agar and incubated overnight at 37°C. The spots were examined for uniformity of the plaques by their morphology, which was individually purified by repeating the process thrice. For each of the purified phage lysates, the overlay agar method was employed to amplify the virus concentrations. The agar overlay plate was flooded with 1 ml of SM buffer and incubated at 4°C for 1 hr. The SM buffer was collected using a syringe and then micro-filtered using 1 0.22μl in a sterile Eppendorf tube and then kept at refrigeration temperature until further analysis. The cocktail was created by combining newly isolated and purified bacteriophages, P01, P03, and P04 in a 1:1:2 ratios [unpublished data]. These bacteriophages were among four phages (Pa01–Pa04) isolated from hospital effluents in western Uganda with distinct plaque morphological appearances on carbapenem-resistant Pseudomonas aeruginosa. The phage cocktails were prepared in three different concentrations so that MOI 1, 10, and 100 contained 2*106, 2*107, and 2*108 plaque forming units per milliliter (PFU/ mL) in comparison to the bacteria’s 2*106 colony forming units per milliliter (CFU/mL), respectively.
Experimental Induction of Wounds in The Swiss Mice
The process of wound induction was done according to the method of [35], with some modifications. Briefly, under local anesthesia (ketamine 80mg/kg body weight), the operative region (dorsum) of all mice was prepared by removing the fur with a hair removal cream (Fem Hair Removal cream, USA) from the base of the neck to 3 cm further down the back and between the two shoulder blades. To avoid post-application irritation and skin dehydration, a pH balancing skin care lotion was applied where the hair had been removed. Two days later, the animals were sedated with ketamine (80-120 mg/kg) and xylazine (5-10 mg/kg) administered by the intraperitoneal route. The backs of the mice were disinfected with 70% (v/v) ethanol. Using a biopsy punch instrument, a 4mm diameter round wound was inflicted through the Panniculus carnosus muscle of the inter-scapular region of the upper back of each mouse. The process was repeated to create another wound on the other side of the midline of the mouse. The two wounds represented the test and control sites for the experiments on each animal.
Contamination of the Induced Wounds in the Swiss Mice
wSilicone splints (made of scar plaster) were centrally placed over the wounds and sutured (with interrupted 6-0 nylon) to securely anchor them after which 50μL of the CRPA bacterial suspension (that is, 1.5*106 CFU/mL) was applied to both wounds. The wounds were covered with an occlusive dressing (plaster) and the mice were randomly divided into five (5) groups each with five (5) animals; the randomization was done as shown in Table 1. After 18 hours, the different wound treatment regimens, as presented in Table 1, were started. The wound treatment comprised of topical application of 50μL of bacteriophage cocktail (Group III-V) and colistin (Colistin sodium methane sulfonate, SIGMA ALDRICH) and meropenem (Meropenem sulfate, SIGMA ALDRICH) for groups II and I as controls, respectively. The treatments were repeated daily for 5 consecutive days and the measurements made for an extra 5 days till the tenth day.
Evaluation of the Effect of the Selected Phage Cocktail on Wound Healing
Using a Vernier caliper, the diameters of the wounds were measured daily and recorded in millimeters till the tenth day. This meant that the experiment took 10 days given that the wounds of mice close up within 5 days [36]. The wound measurements were done from different angles to obtain the average diameter. At the end of the experiment, the mice were anesthetized and then euthanized by injection of sodium pentobarbital and disposed of by incineration.
Results
The daily mean wound diameters as measured for each group are presented in Table 2 for the first, second, fourth, sixth, eighth, and tenth days. The mean wound diameters at the end of the experiment were 3.04, 1.01, 1.14, 1.15, and 1.02 mm for Group I (treated with meropenem), Group II (treated colistin), Group III (treated with MOI 1 of phage cocktail), Group IV (treated with MOI 10 of phage cocktail) and Group V (treated with MOI 100 of phage cocktail), respectively. From day 4 to day 10 of the experiment, there were significant mean differences (p < 0.0001) in the measured wound diameters between the meropenem – and the other treatment groups (Groups II-V). The calculated percentage of wound closure (average) for each group after the last measurement is also given in Table 2. The results depict a sharp increase (Figure 1) in wound closure at day 4. Group II had the biggest wound closure (75.6%) with the bacteriophage cocktail–treated wounds registering satisfactory percentage wound closures of at least 67.7%.
Figure 1: The within-group comparisons between the saline-treated and test substance-treated wounds. A: Group I (10mg/ml meropenem), B: Group II (10mg/ml Colistin), C: Group III (Phage cocktail at MOI: 1), D: Group IV (Phage cocktail at MOI: 10), and Group V (Phage cocktail at MOI: 100).

Table 2: Comparison of the effect of the different bacteriophage concentrations and the standard antibiotics on wound size over time.
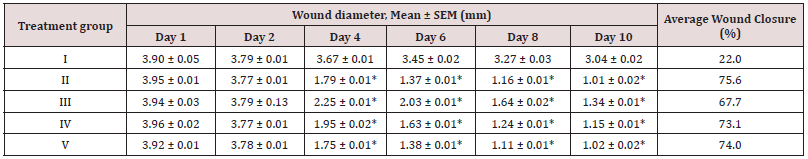
Between-group comparisons amongst wounds treated with one of the test substances over the 10 days of treatment. * - p value < 0.0001. Group I: Meropenem; Group II: Colistin; Group III: MOI of 1; Group IV: MOI of 10; Group V: MOI of 100.
On the other hand, comparisons between the negative controls (saline-treated wounds) and the positive control group with meropenem–treated wounds did not show any significant differences in the wound closure. However, the colistin–treated wounds and those treated with bacteriophage cocktails revealed significant differences in the percentage wound closure as shown in Figure 1. Both the infected saline-treated wounds (negative controls) and meropenem-treated wounds had a decrease in wound size, but they could only produce percentage wound closures at 18.7% and 22.0% by day 10, respectively. The wound closure was observed to increase over time from day one to the tenth day of the experiment. The normal saline-treated wounds were also observed to have some level of healing like that of the meropenem group. However, the normal saline-treated wounds had an insignificant wound healing activity in comparison to the colistin- and phage-treated groups. The wound closure in the latter groups was significant and directly proportional to the duration of the experiment (Figure 1).
Discussion
This study employed a locally formulated 3-bacteriophage combination to evaluate the efficacy of a phage cocktail in healing wounds infected with carbapenem – resistant P. aeruginosa. As a result of the wounding process, the skin’s integrity is compromised, making it easy for any accessible bacteria to enter the interior infection site and cause sepsis due to the contamination [37]. Sepsis can cause either acute or chronic wound failure, depending on the level of care provided or intervention made. In this study, the wounds were infected the same day the wounds were inflicted. Most studies on the therapeutic efficacy of phage application against infectious diseases caused by P. aeruginosa in experimental animal models with the pathophysiology of diseases that closely resemble those in humans have yielded very promising results for future use which this very study has supplemented. The three multiplicities of infection (MOIs) used in this study produced significant wound healing in comparison to that of meropenem implying that even at lower concentrations the cocktail successfully challenged the CRPA.
With an MOI of 3, a percentage of wound closure comparable to that of the last-resort antibiotic, colistin, was observed. The efficacy of bacteriophage cocktails has been studied elsewhere and the results of such reports are in concordance with those of this study [38]. The rise in publications unquestionably reflects a rise in interest among experts in using bacteriophages to treat difficult wounds, especially those in burn patients. This study employed the local application of the bacteriophage cocktail which has been proven extremely successful in the treatment of topical infections, as has the inhalation of phages for the treatment of lung infections [39,40].
It has been argued that in vivo phage concentrations decrease due to clearance mechanisms including interactions with host antibodies and as a result, repeated dosing of phages and sustained release approaches are deemed vital [41]. This is explained by the fact that phages have a tendency to spread throughout the body of the host but are soon entrapped in secondary lymphoid tissues [42].
To improve the lytic activity of the bacteriophages, this study used once-daily dosing of phages for a total treatment period of 5 days. This method ensures that phage concentrations at the site of infection are high enough to result in significant in situ phage amplification, thereby slowing the rate of replication of the target bacteria population. This was aided by the use of an occlusive dressing that prevented cross-contamination and provided optimal conditions for bacterial growth. The study used splints around the wounds to limit the repair process to only epithelialization, angiogenesis, and cellular proliferation, which closely mimics the biological processes of human wound healing other than contraction in mice [43]. As a result, the percentage closure reported in this study is free from the effect of contraction. In as much as the wounds treated with saline exhibited some level of healing with an average closure of 18.7%, this was only comparable to the meropenem-treated wounds.
The mean diameters of the negative controls (wounds treated with normal saline) across the five groups were statistically indifferent in comparison to the meropenem-treated wounds. These findings lead us to the conclusion that this 3-phage cocktail may be more efficacious than or as good as colistin, the common last-resort antibiotic used to treat carbapenem-resistant bacterial infections. The mechanism of action of phages has not been found to be universal for the studies that have attempted to elucidate this issue. However, studies have shown that phages work by inducing the production of cytokines and chemokines, modulating cytokine responses in response to treatment [44] and stimulating low-level immune responses without manifesting any overt symptoms in order to continuously prime innate immune responses [45]. For phages to live up to their potential as a disruptive treatment to combat the rising menace of bacterial infections that are resistant to antibiotics, more preclinical research is required to clarify their specific mechanisms and limitations.
Conclusion
When topically administered in sufficient doses and at optimal frequencies, this study demonstrated the efficacy of a locally formulated and optimized three-phage cocktail in the management carbapenem-resistant Pseudomonas aeruginosa. The results of this study can be used to inform policy and implementation science as an input into the national strategies for combating AMR in Uganda.
Data availability statement
The data used to support the findings of this study have been deposited in the Mendeley Data repository (doi: 10.17632/ p5324rwnkx.1). [dataset] Ibanda, Ivan (2022), “Data for Efficacy Evaluation of a Lytic Bacteriophage Cocktail Against CRPA in a Mouse Wound-infection Model”, Mendeley Data, V1.
Conflicts of Interests
The authors declare no conflict of interest.
Author Contributions
I: JN and CK: Conceptualization, Writing – original draft, Writing – review & editing. II: Data curation, analysis and interpretation. All the authors have read and approved the final version of the manuscript.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
Mr. Kizza Ronald, Laboratory Technician, and Mr. Kamusiime Charles, Laboratory Assistant, both Pharmacology Animal facility at Kampala International University - Western Campus, deserve special gratitude.
References
- Mulani MS, Kamble EE, Kumkar SN, Tawre MS (2019) Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front Microbiol 10(539): 1-24.
- El Zowalaty ME, Thani AA Al, Webster TJ, Zowalaty AE El, Schweizer HP, et al. (2015) Pseudomonas aeruginosa: arsenal of resistance mechanisms, decades of changing resistance profiles, and future antimicrobial therapies. Future Microbiol 10(10): 1683-1706.
- Kortright KE, Chan BK, Koff JL, Turner PE (2019) Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 25(2): 219-232.
- Wienhold SM, Lienau J, Witzenrath M (2019) Towards Inhaled Phage Therapy in Western Europe. Viruses 11(3): 295.
- Burrowes B, Harper DR, Anderson J, Mcconville M, Enright MC (2011) Bacteriophage therapy: potential uses in the control of antibiotic-resistant pathogens. Expert Rev Anti Infect Ther 9(9): 775-785.
- Furfaro LL, Payne MS, Chang BJ (2018) Bacteriophage Therapy: Clinical Trials and Regulatory Hurdles. Front Cell Infect Microbiol 8: 376.
- Nagel TE (2018) Delivering Phage Products to Combat Antibiotic Resistance in Developing Countries: Lessons Learned from the HIV/AIDS Epidemic in Africa. Viruses 10(7): 345.
- Szafranski S, Winkel A, Stiesch M (2017) The use of bacteriophages to biocontrol oral biofilms. J Biotechnol 250: 29-44.
- Keen EC (2014) A century of phage research: Bacteriophages and the shaping of modern biology. Bioessays Journal 37(1): 6-9.
- Latz S, Wahida A, Arif A, Hafner H, Hoß M, et al. (2016) Preliminary survey of local bacteriophages with lytic activity against multi-drug resistant bacteria. J Basic Microbiol 56(10): 1117-1123.
- Hraiech S, Brégeon F, Rolain JM (2015) Bacteriophage-based therapy in cystic infections : rationale and current status. Drug Des Devel Ther 9: 3653-3663.
- Soothill J (2013) Use of bacteriophages in the treatment of Pseudomonas aeruginosa infections. Expert Rev Anti Infect Ther 11(9): 909-915.
- Budzik JM, Rosche WA, Rietsch A, Toole GAO (2004) Isolation and Characterization of a Generalized Transducing Phage for Pseudomonas aeruginosa Strains PAO1 and PA14. J Bacteriol 186(10): 3270-3273.
- Ceyssens PJ, Lavigne R, Mattheus W, Chibeu A, Hertveldt K, et al. (2006) Genomic Analysis of Pseudomonas aeruginosa Phages LKD16 and LKA1 : Establishment of the phi-KMV Subgroup within the T7 Supergroup. J Bacteriol 188(19): 6924-6931.
- Heo Y, Lee Y, Jung H, Lee J, Ko G, et al. (2009) Antibacterial Efficacy of Phages against Pseudomonas aeruginosa Infections in Mice and Drosophila melanogaster. Antimicrob Agents Chemother 53(6): 2469-
- Salman AE, Abdulamir AS (2014) Assessment of bacteriophage cocktails used in treating multiple-drug resistant Pseudomonas aeruginosa. Int J Curr Microbiol Appl Sci 3(11): 711-722.
- Watanabe R, Matsumoto T, Sano G, Ishii Y, Tateda K, et al. (2007) Efficacy of Bacteriophage Therapy against Gut-Derived Sepsis Caused by Pseudomonas aeruginosa in Mice. Antimicrob Agents Chemother 51(2): 446-
- Forti F, Roach DR, Cafora M, Pasini ME, Horner DS, et al. (2018) Design of a Broad-Range Bacteriophage Cocktail That Reduces Pseudomonas aeruginosa Biofilms and Treats Acute Infections in Two Animal Models. Antimicrob Agents Chemother 62(6): e02573-e02617.
- Mcvay CS, Vela M, Fralick JA (2007) Phage Therapy of Pseudomonas aeruginosa Infection in a Mouse Burn Wound Model. Antimicrob Agents Chemother 51(6): 1934-1938.
- Mendes J, Leandro C, Mottola C, Barbosa R, Silva FA, et al. (2014) In vitro design of a novel lytic bacteriophage cocktail with therapeutic potential against organisms causing diabetic foot infections. J Med Microbiol 63: 1055-1065.
- Vieira A, Silva YJ, Cunha Â, Gomes NCM, Al E (2012) Phage therapy to control multidrug-resistant Pseudomonas aeruginosa skin infections : in vitro and ex vivo experiments. Eur J Clin Microbiol Infect Dis 31(11): 3241-3249.
- Waters E, Neill D, Kaman B, Al E (2017) Phage therapy is highly effective against chronic lung infections with Pseudomonas aeruginosa. Thorax 72(7): 666-667.
- Jennes S, Merabishvili M, Soentjens P, Pang KW, Rose T, et al. (2017) Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury-a case report. Crit Care 21(1): 129.
- Law N, Logan C, Yung G, Furr CLL, Lehman SM, et al. (2019) Successful adjunctive use of bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa infection in a cystic fibrosis patient. Infection 47(4): 665-668.
- Morozova W, Tikunova NV (2018) Applications of Bacteriophages in the Treatment of Localized Infections in Humans. Front Microbiol 9: 1696.
- Nale JY, Redgwell TA, Millard A, Clokie MRJ (2018) Efficacy of an Optimised Bacteriophage Cocktail to Clear Clostridium difficile in a Batch Fermentation Model. Antibitotics 7(1): 13.
- Nasr-Eldin MA, El-Maaty SAA, El-Dougdoug KA, Hazaa MM (2018) Characterization and development of a phage cocktail for Escherichia coli causing gastrointestinal diseases. J Bas Environ Sci 5: 115-122.
- Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM (2011) Phage treatment of human infections. Bacteriophage 1(2): 66-85.
- Nagel TE, Chan BK, Vos D De, El-shibiny A, Kang’ethe EK, et al. (2016) The Developing World Urgently Needs Phages to Combat Pathogenic Bacteria. Front Microbiol 7: 882.
- Semler DD, Lynch KH, Dennis JJ (2012) The promise of bacteriophage therapy for Burkholderia cepacia complex respiratory infections. Front Cell Infect Microbiol 1: 27.
- Kateete DP, Nakanjako R, Okee M, Joloba ML, Najjuka CF (2017) Genotypic diversity among multidrug resistant Pseudomonas aeruginosa and Acinetobacter species at Mulago Hospital in Kampala, Uganda. BMC Res Notes 10(1): 284.
- Elliot S, Wikramanayake TC, Jozic I, Tomic-Canic MA (2018) A Modeling Conundrum: Murine Models for Cutaneous Wound Healing. J Invest Dermatol 138(4): 736-740.
- Clokie Martha Kropinski, Andrew Lavigne Rob (2018) Bacteriophages: Methods and Protocols, Volume 3. Methods Mol Biol 1681.
- Bhensdadia D, Bhimani H, Nathani N, Rawal C (2014) Isolation, Molecular Characterization and Insight into the Genome Sequence Next Generation : Sequencing Applications Isolation. Molecular Characterization and Insight into the Genome Sequence of E . coli Bacteriophage ADB-2 from Poultry Fecal Sample. Next Generation Sequencing Application 1(1): 1-7.
- Dunn L, Prosser HCG, Tan JTM, Vanags LZ, Ng MKC, et al. (2013) Murine Model of Wound Healing. J Vis Exp 28: 75.
- Grada A, Mervis J, Falanga V (2019) Research Techniques Made Simple: Animal Models of Wound Healing. J Invest Dermatol 138(10): 2095-2105.
- Leontyev АE, Pavlenko IV, Kovalishena ОV, Saperkin NV, Tulupov АА, et al. (2021) Application of Phagotherapy in the Treatment of Burn Patients (Review). Sovrem Tekhnologii Med 12(3): 95-103.
- Pires DP, Boas DV, Sillankorva S, Azeredo J (2015) Phage Therapy: a step Forward in the Treatment of Pseudomonas aeruginosa Infections. J Virol 89(15): 7449-7456.
- Qadir MI, Mobeen T, Masood A (2013) Phage therapy : progress in pharmacokinetics. Braz J Pharm Sci 54(1): 1-9.
- Ryan EM, Gorman SP, Donnelly RF, Gilmore BF (2011) Recent advances in bacteriophage therapy: how delivery routes , formulation , concentration and timing influence the success of phage therapy. J Pharm Pharmacol 63(10): 1253-1264.
- Malik DJ, Sokolov IJ, Vinner GK, Mancuso F, Cinquerrui S, et al. (2017) Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv Colloid Interface Sci 249: 100-133.
- Rouse MD, Stanbro J, Roman JA, Lipinski MA, Jacobs A, et al. (2020) Impact of Frequent Administration of Bacteriophage on Therapeutic Efficacy in an A. baumannii Mouse Wound Infection Model. Front Microbiol 11: 414.
- Thompson MG, Black CC, Pavlicek RL, Honnold CL, Wise MC, et al. (2014) Validation of a novel murine wound model of Acinetobacter baumannii Infection. Antimicrob Agents Chemother 58(3): 1332-1342.
- Park K, Cha KE, Myung H (2014) Observation of inflammatory responses in mice orally fed with bacteriophage T7. J Appl Microbiol 117(3): 627-633.
- Duerkop B, Hooper AH (2013) Resident viruses and their interactions with the immune system. Nat Immunol 14(7): 654-659.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...





