
Lupine Publishers Group
Lupine Publishers
Menu
Review Article (ISSN: 2770-5447)
Problems of Biomineralization Dissolution in Human Arteries Volume 1 - Issue 4
Maciej Pawlikowski*
- AGH University Science and Technology, Cracow, Poland
Received: October 01, 2018; Published: October 09, 2018
Corresponding author: Maciej Pawlikowski, AGH University Science and Technology, Cracow, Poland
DOI: 10.32474/ACR.2018.01.000118
Abstract
Keywords: Artery biomineralization; Dissolution
Introduction
Plenty has been written about the impact of arteriosclerosis, i.e. arterial biomineralization, on the functioning of human body. A little less information is available concerning the dissolution and prevention of such mineralization (calcium channel blockers etc.) [1,2]. The significance of even a small progress in “cleaning” the arteries cannot be overstated. By renewing the proper functioning of the cardiovascular system, it will restore proper functioning of tissue, organs, and other elements of the body, and in effect support removal or reduction of many diseases. It will lead to lasting rejuvenation of the body.
Methods
Two types of mineralization present in arteries are mineralization with organic compounds (Figure 1) and/or mineralization with inorganic phosphorus compounds (Figure 1). However, the most common is organic-inorganic mixed mineralization with varying proportions of both types [3]. That hinders dissolution of deposits (arteriosclerotic plaque), because organic solvents are needed to dissolve organic deposits, while inorganic deposits require inorganic solvents. In the most common case of mixed arteriosclerotic plaque, combinations of organic and inorganic solvents need to be used [4-8].
Figure 1: A – concentration of cholesterol in arterial wall (arrows), B – cholesterol crystal aggregates formed on the surface of the intima, C – apatite crystal aggregates on the surface of the intima (arrows indicate places where chemical analyzes were carried out using EDS), D – phosphate aggregates on the intima surface.
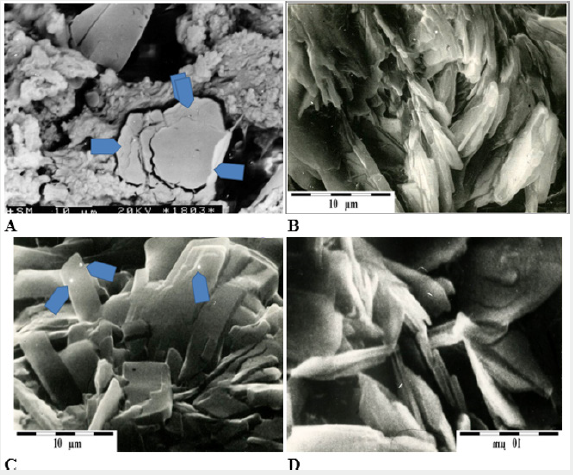
Mineralization develops in biomineralization centers, which are places where different elements of artery structure have been damaged (39). Arteriosclerotic plaque is built from components transported with blood — mostly cholesterol, free Ca2+ and PO43- ions, etc. It is interesting to note that biomineralization of both the intima and the arterial wall is not related to exceeding the solubility product of crystallizing components [9], because concentrations of those components in blood (mainly in plasma) are low. Since the formation of deposits occurs even at low concentrations of those substances in blood, it means that the mechanism that governs the biomineralization of arteries is slightly different than the one occurring after exceeding the so-called solubility product of crystallizing substance [10], where the concentration of components is so high that they must precipitate out of the transporting medium.
This situation causes an additional problem with the dissolution of deposits formed in the arteries. It is impossible to shift the system of chemical equilibrium in the blood in such a way that the number of components we want to dissolve from previously crystallized biomineralization is very low. Although that would undoubtedly promote the dissolution of deposits [11-16], it may disrupt the functioning of other organs in which circulating blood must contain a certain amount of the listed ingredients [17,18]. Considering the above, it was decided to first attempt to dissolve the deposits in vitro, and then, in the future, use the obtained test results in attempts to dissolve arterial biomineralization in vivo (Figure 2).
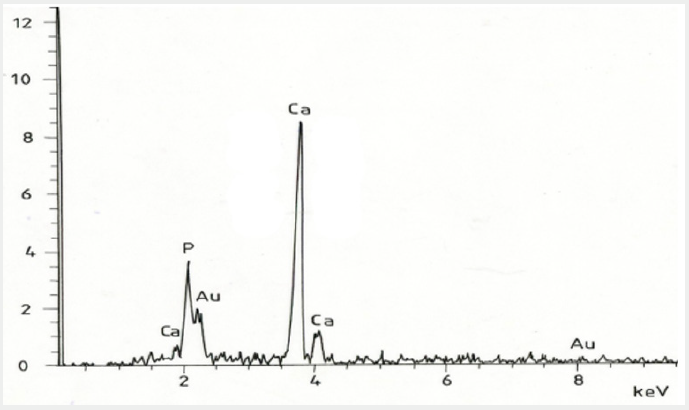
Figure 2: EDS energy spectrum of phosphate from the intima surface shown in Photo 1 D.
Experimental Dissolution – Equipment and Method
The apparatus shown in Figure 2 was used for experimental dissolution of arterial biomineralization in vitro. It consists of a container with solvent (1), a peristaltic pump (2), a fragment of artery with cholesterol biomineralization or a tube with synthetic calcification (3), and a reservoir for solvent after its passage through an artery or tube with synthetic apatite (4). The solvent flow rate was adjusted to the average blood flow rate in human body [19- 25]. The experiment was carried out at a temperature of 22 °C, in normal atmospheric pressure.
Figure 3: Drawing of a set of devices used in the experimental dissolution of arterial biomineralization. 1 - container with solvent, 2 - peristaltic pump, 3 - fragment of artery with cholesterol biomineralization or tube with synthetic calcifications, 4 - container for solvent after it flows through the system (4). Arrows show the direction of solvent flow.
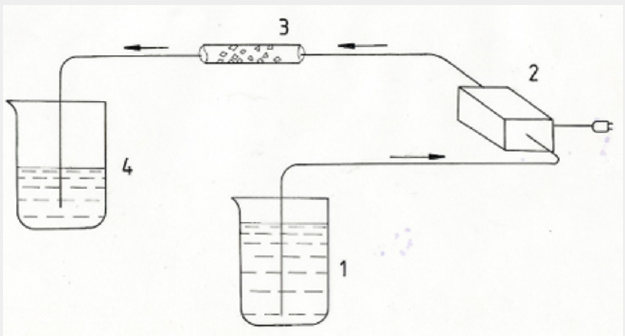
The experiment included both mineralization occurring in arteries and synthetic apatite mineralization placed in a tube (Figure 3). Testing was carried out before and after passing the solvent through. Tests were also carried out on the products of crystallization from the solvent after it had passed through the biomineralization zone [25-31]. Polarization microscope (Meiji microscope manufactured in China) and scanning microscope (Jeol 540 and 560 microscope of Japanese production) were used in the research [32-36].
Results of the experiment are presented as follows:
- Dissolution of organic biomineralization
- Dissolution of phosphate (apatite) mineralization
Dissolution of organic biomineralization
Dissolution of organic deposits occurring on walls of arteries used in experiments was preceded by tests regarding presence of cholesterol deposits [36-41]. Many different solvents were passed through the arteries (Figure 4), but only the results of the best dissolution effects were presented in this publication. Fragments of arteries were obtained due to the kindness of cardiac surgeon prof. Roman Pfitzner from the John Paul II Hospital in Krakow, for which the author gives heartfelt thanks [42]. Ethyl alcohol proved to be one of the best cholesterols and fats solvents. After pumping through the arteries, it was evaporated, and the resulting microcrystals were examined. The studies showed that the crystals were represented by microcrystalline cholesterol (Figure 5).
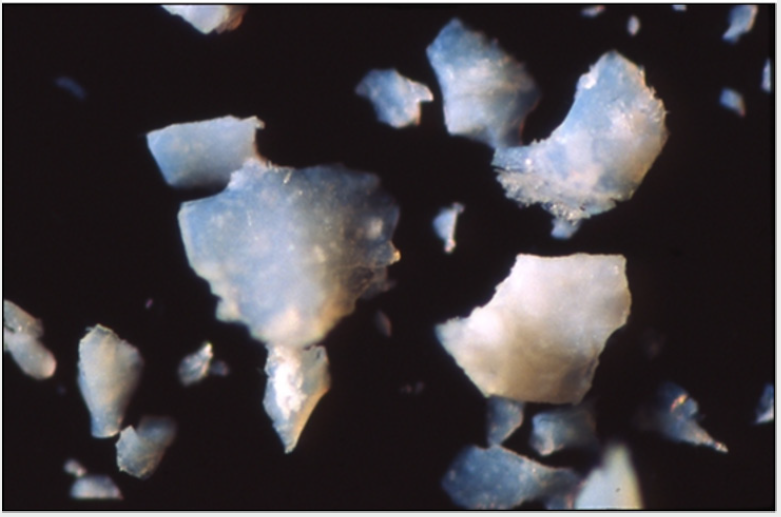
Figure 4: Fragment of an artery affected by significant cholesterol biomineralization, used in the experiments (arrows).

Figure 5: Cholesterol microcrystal crystallized out of the ethanol that was pumped through arteries affected by cholesterol biomineralization.

Dissolution of phosphate (apatite) mineralization
Experiments with dissolving phosphate biomineralization proved to be complicated, because in the studied arteries such mineralization never occurred without accompanying cholesterol. In order not to complicate the experiment [43-49], it was decided that apatite would be synthesized, and an “artificial” artery covered by phosphate mineralization would be created. Arterial phosphate biomineralization [50], which in previous studies proved to be common (although usually co-occurring with organic compounds), turned out to be very complicated to synthesize outside the body. Many experiments allowed us to obtain a phosphate gel, which during crystallization led to the synthesis of microcrystalline carbonate apatite where about 10% of PO43- groups were replaced by CO32– groups (Figure 6). Size of the crystals of this apatite was regulated through the time of crystallization [51,52], which was carried out at the temperature of 30 °C. Mineralogical studies confirmed total mineralogical compatibility of the obtained synthetics with apatite formed in the arteries.
Figure 6: Fragments of microcrystalline apatite obtained through experimental synthesis and crystallization of phosphate gel.
Grains of the synthetic apatite were placed inside a plastic tube, which was then attached to the experimental dissolution system (Figure 7 & 8). Experimental dissolving of synthetic apatite was attempted with many solvents. The best one proved to be distilled water [53-56]. The surface of apatite grains removed from the tube was studied after their experimental dissolution with distilled water as a solvent. Observations conducted with scanning microscopy confirmed dissolution of apatite grains. It manifested on their surface as a series of morphological depressions, channels and irregularities (Figure 9).
Figure 7: Photomicrograph of the apatite structure obtained through the synthesis of phosphate gel, which was used in experimental dissolution SEM.
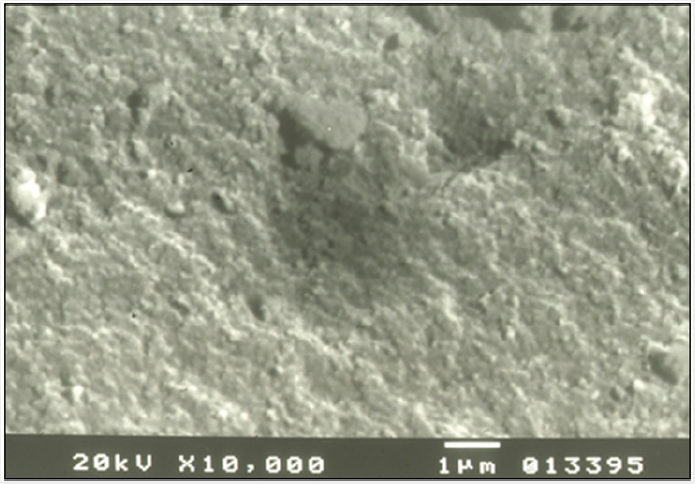
Figure 8: Tube with grains of synthetic apatite (arrows) attached to the experimental arterial biomineralizetion dissolution system.
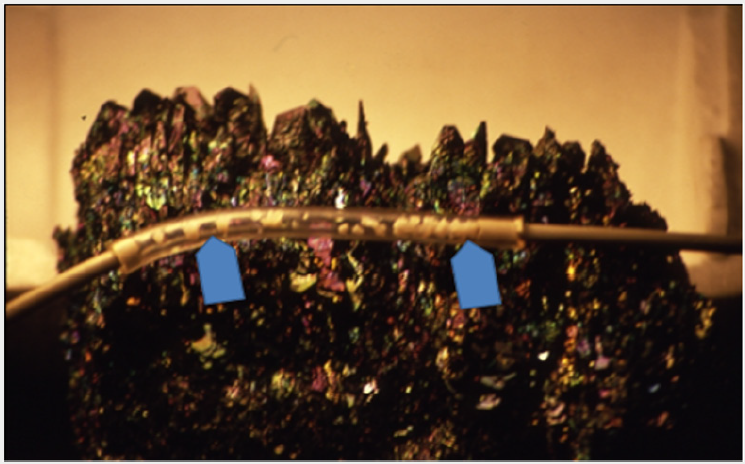
Figure 9: Surface of dissolved grain of synthetic apatite after experimental dissolution with distilled water. SEM.

Summary
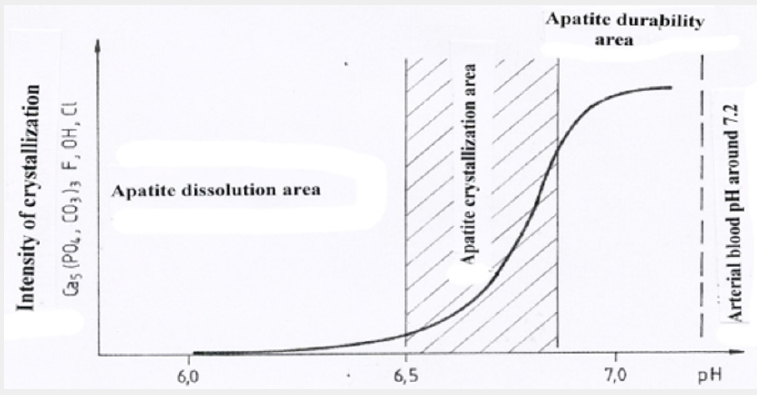
Presented research results are an experimental contribution to better understanding of the phenomena of crystallization and dissolution of arterial biomineralization, which is one of the main research problems concerning the circulatory system. The experiments that were carried out indicate the possibility of dissolving both cholesterol and apatite deposits found in human arteries. Depending on the type of arterial biomineralization, different “solvents” should be used: organic solvents for organic substances, inorganic for apatite deposits [57]. It is probably also possible to use mixed composition solvents that will dissolve the most common type of arterial biomineralization, i.e. organicinorganic mixed mineralization. However, that requires further experimental research. Experimental synthesis of apatite needed for presented research allowed us to determine the conditions of both its crystallization and dissolution (Figure 10). They explain why apatite deposits do not form in veins [58]. Venous blood contains CO2 and other products of metabolism that cause its slight acidification. Lower pH is enough to prevent phosphates, including apatite, crystallizing in the veins. The range of apatite instability is pH 6.6-6.8.
Figure 10: Diagram of the pH balance of the solution - experimental crystallization and dissolution of calcium apatite with some of PO43- groups replaced by CO32- as well as F- , Cl- and OH- ions.
Since it is impossible to lower the arterial blood pH to such values, other elements should be considered when thinking about dissolution of arterial biomineralization [59-61]. In case of calcium phosphates, the body should be stimulated in such a way that the level of ionized calcium (Ca2+) in the blood plasma is reduced (without detriment to the body’s functioning). It appears that thyroid and the parathyroid glands may play an important role here. Situation with cholesterol as a derivative of bile acids synthesized by the liver is more difficult. Shifting the chemical balance of arterial blood towards lowering the level of cholesterol does not eliminate the cause, which is dysfunction of the liver that causes intense cholesterol synthesis. It seems important to stimulate the liver in such a way as to reduce the amount of bile acid in the bile, which is responsible for the synthesis of cholesterol and its transport in the bloodstream. The problem is complex and requires further multidirectional research.
References
- Lim J, Tang TB, Park D, Demer LL, Abedin M, et.al. (2006) N-3 fatty acids inhibit vascular calcification via the p38-mitogen-activated protein kinase and peroxisome proliferator-activated receptor-gamma pathways. 98(6): 727-729.
- Allison MM, Criqui MH, Wright CM (2004) Patterns and risk factors for systemic calcified atherosclerosis. 24(2): 331-336.
- Arad Y, Goodman KJ, Roth M, Newstein D (2005) Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. 46(1): 158- 65.
- Lopez I, Perez J, Rodriguez M, Aguilera Tejero E (2006) Reversibility of calcitriol-induced medial artery calcification in rats with intact renal function. J Bone Miner Res 21(3): 484-490.
- Bostrom K, J Clin Invest, Watson KE, Horn S, Wortham C, Herman IM, et al. (1993) Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest 91(4): 1800-1809.
- Callaway MP, Br J Radiol, Richards P, Goddard P, Rees M (1997) The incidence of coronary artery calcification on standard thoracic CT scans. 70(834): 572-574.
- Campbell GR, Z Kardiol, Campbell JH (2000) Vascular smooth muscle and arterial calcification 89: 54-62.
- Canfield AE, Sutton AB, Hoyland JA, Schor AM (1996) Association of thrombospondin-1with osteogenic differentiation of retinal pericytes in vitro. J Cell Sci 19(2): 343-353.
- Cowell SJ, N Engl, J Newby DE, Prescott RJ, Bloomfield P, Reid J, et al. (2005) A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. 352(23): 2389-2397.
- El Abbadi M, Giachelli CM (2007) Mechanisms of vascular calcification. Adv Chronic Kidney Dis 14 (1): 54-66.
- Huang H, Virmani R, Younis H, Burke AP, Kamm RD, et al. (2001) The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation 103(8): 1051-1056.
- Iribarren C, Sidney S, Sternfeld B, Browner W S (2000) Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA 283(21): 2810-2815
- Jono S, McKee MD, Murry C E, Shioi, Nishizawa Y, et al. (2000) Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87(7): E10-E17.
- Keelan PC, Bielak LF, Ashai K, Jamjoum LS, Denktas AE, et al. (2001) Long-term prognostic value of coronary calcification detected by electron-beam computed tomography in patients undergoing coronary angiography. Circulation 104(4): 412-417.
- Lomashvili KA, Hennigar RA, Hardcastle KI, Oneill WC (2004) Phosphateinduced vascular calcification: role of pyrophosphate and osteopontin J Am Soc Nephrol 15(6): 1392-1401.
- Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, et al. (1997) Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 6386(6620): 78-81.
- Matsushita M, Nishikimi N, Sakurai T, Nimura Y (2000) Relationship between aortic calcification and atherosclerotic disease in patients with abdominal aortic aneurysm. Int Angiol 19(3): 276-279.
- Mason JC, Philippidis P, Florey O, Smythe CD,Landis, et al. (2005) Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: a vicious cycle of inflammation and arterial calcification? Circ Res 96(123): 1248-1256.
- Neves KR, Graciolli FG, Dos Reis LM, Graciolli RG, et al. (2007) Vascular calcification: contribution of parathyroid hormone in renal failure. Kidney Int 71(21): 1262-1270.
- Nicholls SJ, Tuzcu EM, Wolski K, Sipahi I,Nissen ES, et al. (2007) Coronary artery calcification and changes in atheroma burden in response to established medical therapies. J Am Coll 49(2): 263-270.
- Niskanen L, Siitonen O, Suhonen M, Uusitupa MI (1994) Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care 17(11): 1252
- Orita Y, Yamamoto H, Kohno N, Sugihara M, Mito S, et al. (2007) Yoshizumi M. Role of osteoprotegerin in arterial calcification: development of new animal model. Arterioscler Thromb Vasc Biol 27(9): 2058-2064.
- Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, et al. (1997) Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation: a possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol 17(4): 680-687.
- Parhami F, Basseri B, Hwang J, Tintut Y (2002) High-density lipoprotein regulates calcification of vascular cells. Circ Res 91(7): 570-576.
- Pawlikowski M (1987) (Mineralization of human living organism). Prace Mineral 79: 121.
- Pawlikowski M, Ryskala Z (1991) Mineralogical and chemical characterization of phosphate mineralization of selected arterial vessels. (Mineralogical-chemical characteristic of phosphate mineralization of human arteries). Annals of Sciences Dyd, WSP in Krakow. Physiological works pp. 81-104.
- Pawlikowski M (1991) Mineralization of cancer In: Biomineralisation and biomaterials. PWN, Warsaw pp. 84-92.
- Pawlikowski M (1993) Crystals in the human body (Crystals of humanorganism). Secesja pp. 132.
- Pawlikowski M (1995) Secrets of tissue mineralization. (Secrets of tissue mineralization). PAN Cracow pp. 97.
- Pawlikowski M (1999) Preliminary results of dissolution of substances mineralizing human arteries. Arch Mineralog 52: 195.
- Pawlikowski M, Pfitzner R (1995) Application of mineralogical methods in human tissue research. I Ways to study mineralization (Mineralogical methods useful for examination of human tissues). Przegl Lekarski 52: 119-123.
- Pawlikowski M, Pfitzner R (1995) Application of mineralogical methods in human tissue research. II. Mineralization of heart structures (Mineralogical methods useful for examination of human tissues. Mineralization of heart structures). Przegl Lekarski 52: 24-27.
- Pawlikowski M, Pfitzner K, Skinner C (1995) Cholesterol-mineral concentrations of the aneurysmatic wall. Acta Angiologica pp. 1-15.
- Pawlikowski M, Pfitzner R (1999) Mineralization of heart, (Mineralization of heart and big blood vessels). Wyd IGSMiE PAN Kraków pp. 142
- Pawlikowski M (2003) Minerals in human blood vessels and their dissolution in vitro. In: Skinner HCW, Berger AW, editors. Geology and health. Oxford University Press pp. 155-158.
- Pawlikowski M (2011) Biomineralization of cancer tissues 20th Int Symp Molecular and Physiological Aspects of Regulatory Processes of the Organism. Cracow, H Lach editors. Wyd Abaton Kraków pp. 190-191.
- Pawlikowski M (2014) Osteoporosis as a source of tissue mineralization. Research on osteoporosis therapy and dissolution of arterial mineralization. Jour Life Science 8: 610-625.
- Pawlikowski M (2017) Biomineralogy of angiogenesis. Arch Clin Biomed Res 1: 161-167.
- Pawlikowski M (2017) a Centers of Human Tissue Biomineralization (Calcification). Cardiol. Cardiovasc Med 1: 252-262.
- Price PA, Faus SA, Williamson MK (2000) Warfarin-induced artery calcification is accelerated by growth and vitamin D. Arterioscler Thromb Vasc Biol 20(2): 317-327.
- Price PA, June HH, Buckley JR (2001) Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol 21(10): 1610-1616.
- Price PA, Faus SA, Williamson MK (2001) Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arterioscler Thromb Vasc Biol 21(5): 817-824.
- Proudfoot D, Skepper JN, Shanahan CM, Weissberg PL (1998) Calcification of human vascular cells in vitro is correlated with high levels of matrix Gla protein and low levels of osteopontin expression. Arterioscler Thromb Vasc Biol 18(3): 379-388.
- Qiao JH, Xie PZ, Fishbein MC, Kreuzer J,Demmer LL, et al. (1994) Pathology of atheromatous lesions in inbred and genetically engineered mice: genetic determination of arterial calcification. Arterioscler Thromb Vasc Biol 14(9): 1480-1497.
- Qiao JH, Fishbein MC, Demer LL, Lusis AJ (1995) Genetic determination of cartilaginous metaplasia in mouse aorta 15(2): 2265-2272.
- Qiao JH, Mertens RB, Fishbein MC, Geller SA (2003) Cartilaginous metaplasia in calcified diabetic peripheral vascular disease: morphologic evidence of enchondral ossification. Hum Pathol 34(4): 402-407.
- Rajamannan NM, Subramaniam M, Stock SR, Stone NJ, Bonow, McConnel, et al. (2005) Atorvastatin inhibits calcification and enhances nitric oxide synthase production in the hypercholesterolaemic aortic valve. Heart 91(6): 806-810.
- Reaven PD, Sacks J (2005) Coronary artery and abdominal aortic calcification are associated with cardiovascular disease in type 2 diabetes, Diabetologia 48(2): 379-385.
- Rennenberg RJ, Schurgers L, Stehouwer CDA (2010) Arterial calcifications. J Cell Mol Med 14(9): 2203-2210.
- Reynolds JL, Joannides AJ, Skepper JN, Shanahan CM, McNair, et al. (2004) Human vascular smooth muscle cells undergo vesiclemediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Cell Mol Med 15(11): 2857-2867.
- Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, et al. (1998) Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol 31(1): 126-133.
- Shanahan CM, Proudfoot D, Farzaneh Far A, Weissberg PL (1998) The role of Gla proteins in vascular calcification. Crit Rev Eukaryot Gene Expr 8(3-4): 357-375.
- Shao JS, Cheng SL, Charlton Kachigian N, Loewy AP, Towler DA, et al. (2003) Teriparatide (human parathyroid hormone (1-34) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J BiolChem 12,278(50): 50195-50202.
- Steitz SA, Speer MY, McKee MD, Liaw L,Yang H, et al. (2002) Steopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol 161(6): 2035-2046.
- Schmermund A, Achenbach S, Budde T, Buziashvili Y,Lahiri A, et al. (2006) Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, doubleblind trial. Circulation 113(3): 427-437.
- Nimura A, McGregor DH, Anderson HC (1983) Soc Exp Proc Soc Exp Biol Med 172(2): 173-177.
- Tse J, Martin-McNaulty B, Halks-Miller M, Kauser K, Rubanyi, et al. (1999) Accelerated atherosclerosis and premature calcified cartilaginous metaplasia in the aorta of diabetic male Apo E knockout mice can be prevented by chronic treatment with 17 beta-estradiol. Atherosclerosis 144(2): 303-313.
- Wada T, McKee MD, Steitz S, Giachelli CM (1999) Calcification of vascular smooth muscle cell cultures: inhibition by osteopontin. Circ Res 84(2): 166-178.
- Wang L, Jerosch-Herold M, Detrano R, Folsom AR, Folsom AR, et al. (2006) Coronary artery calcification and myocardial perfusion in asymptomatic adults: the MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 48(5): 1018-1026.
- Weiss RM, Ohashi M, Miller JD, Young SG (2006) Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation 114(19): 2065- 2069.
- Vliegenthart R, Oudkerk M, Hofman A, Oei HH, Rooij FJ, et al. (2005) Coronary calcification improves cardiovascular risk prediction in the elderly Circulation 112(4): 572-577.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...


