
Lupine Publishers Group
Lupine Publishers
Menu
Research Article(ISSN: 2770-5447)
Platelets and Patent Ductus Arteriosus: Is there a Any Association Really? Volume 2 - Issue 2
Birol Karabulut1*, Ayse Simsek2 and Baran Cengiz Arcagok3
- 1Izmir Katip Celebi University Ataturk Training and Research Hospital, Pediatrics, Division of Neonatology, Karabaglar, Turkey
- 2Buca Gynaecology and Pediatrics Hospital, Turkey
- 3Acibadem Mehmet Ali Aydinlar University, Depatment of Pediatrics, Division of Neonatology, Altunizade, Istanbul, Turkey
Received: May 25, 2019; Published: June 03, 2019
Corresponding author: Birol Karabulut, Izmir Katip Celebi University Ataturk Training and Research Hospital, Pediatrics, Division of Neonatology, Karabaglar, Turkey
DOI: 10.32474/ACR.2019.02.000133
Abstract
Introduction: A great number of the studies have shown that platelets play a role in closure of the PDA. However, studies that reported that platelet parameters were not associated with PDA were also published. We also wanted to contribute to clarify the relationship between PDA and platelet parameters.
Materials and Methods: Preterm infants that less than 34 gestational weeks were examined to echocardiography at the time of detected clinical findings or within 24-72 h after admission to our unit, routinely. The patients were divided into two groups according to echocardiography findings randomly; hsPDA require ductal closure treatment and non-hsPDA. The platelet count, MPV, PDW, PCT and Platelet Mass Index values of both groups were compared.
Results: There was no difference between the two groups in terms of MPV, Platelet count and Platelet mass index. However, PDW and PCT were statistically significantly in the study group than the control group.
Discussion: As a result, according to our study, platelet count, MPV and platelet mass index cannot be used to predict either hsPDA or treatment success, but a low PCT and high PDW can be used predict hsPDA but not treatment success.
Introduction
Patent Ductus Arteriosus (PDA) can cause mortality and morbidity such as respiratory distress syndrome (RDS), pulmonary hemorrhage, bronchopulmonary dysplasia (BPD), intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP) [1]. For this reason, early diagnosis and treatment of PDA is the most important point. The main diagnostic method of PDA is Doppler echocardiography [2]. However, there is no clearly consensus on diagnosis of hemodynamically significant patent ductus arteriosus (hsPDA). Therefore, new diagnostic methods of PDA are needed. A great number of the studies have shown that platelets play a role in closure of the PDA [3-6]. The first of these studies, Echtler et al. studied the relationship between ductal closure and platelet parameters in animals [3]. In the same study, the ductus arteriosus did not close (thus, remained permanently open) in animals in which platelet functions were compromised. After this study, they studied on premature infants about relationship between ductal closure and platelet parameters. According to this study, a low platelet count and a low PDW were risk factors for PDA. However, studies that reported that platelet parameters were not associated with PDA were also published [7-11]. We also wanted to contribute to clarify the relationship between PDA and platelet parameters.
Materials and Methods
This observational, retrospective cohort study was conducted between August 2017 and 2018. Preterm infants that less than 34 gestational weeks were examined to echocardiography at the time of detected clinical findings or within 24-72 h after admission to our unit, routinely [12]. The patients were divided into two groups according to echocardiography findings randomly; hsPDA require ductal closure treatment and non-hsPDA. hsPDA was defined when at least one of the clinical findings associated with PDA was present: a hyperdynamic precordium; a sustained murmur; tachycardia; hypotension; oliguria; an increased pulse pressure; an increase in ventilation pressure and/or oxygen demand; and at least one echocardiographic finding: ductal diameter ≥1.5mm, left atrium/ aortic root ratio ≥1.5, and/or diastolic flow failure in the abdominal aorta or inverse flow. We applied intravenous or oral ibuprofen to close the hsPDA. Intravenous or oral paracetamol was given in cases who ibuprofen is unsuccessful or contraindicated. After treatment, echocardiography was performed again, and the PDA was classified as open or closed. We excluded those with conditions that might cause inflammation or affect platelet count and/or function (Antenatal steroid use, PPROM, early sepsis, chorioamnionitis, congenital viral infections, preeclampsia), congenital heart disease, pulmonary hypertension, perinatal asphyxia, congenital anomaly, chromosomal anomaly, thrombocytopenia (<50.000/mm3), and lack of data. Written informed consent was obtained from all parents. All echocardiographic examinations were performed by Vivid S6 Echocardiography System fitted with a 10S transducer (General Electric Healthcare, Milwaukee, WI, USA). Blood samples taken from an umbilical venous catheter at between 48-72 hours and 7. day, were collected in ethylenediaminetetraacetic acid-containing tubes and blood counts performed using a Coulter Counter model LH (Coulter Electronics, Hialeah, FL, USA). This yielded the platelet count, MPV, PDW, PCT. The platelet mass index was obtained from the platelet count (103/mm3) and the MPV (fL). We recorded gestational age, birth weight, sex, mode of delivery, Apgar scores (at 1 and 5 min) 48-72 h and 7. day platelet parameters, surfactant requirement, ventilation history, IVH, NEC, ROP, BPD, duration of hospitalization and any death.
Statistical Analysis
Statistical analyses were performed using SPSS for Windows ver. 22.0 (SPSS Inc., Chicago, Illinois). The paired samples t-test and independent samples t-test were used to compare continuous variables. Continuous variables are presented as means ± SDs, and categorical variables are given as frequencies with percentages. A p-value less than 0.05 was considered statistically significant.
Results
258 newborns under 34 weeks were admitted to our unit, of whom 121 were excluded. The study group consisted of 72 premature infants with hsDPA who applied ductus closure treatment and 65 premature infants without hs DPA or spontaneously closed PDA consisted of the control group (Figure 1). The demographic characteristics of both groups are shown in Table 1. The mean gestational age and the mean birth weight of the study and control groups were, respectively, 31.4±3.8 vs. 32.3±4.5 weeks (p=0.12); 1441±347 vs. 1,539±286g (p=0.08). There was no difference between two groups in perinatal parameters. The platelet parameters of both groups are shown in Table 2. There was no difference between the two groups in terms of MPV, Platelet count and Platelet mass index. However, PDW and PCT were statistically significantly in the study group than the control group.
Table 1: Comparison perinatal characteristics of the study and control groups.
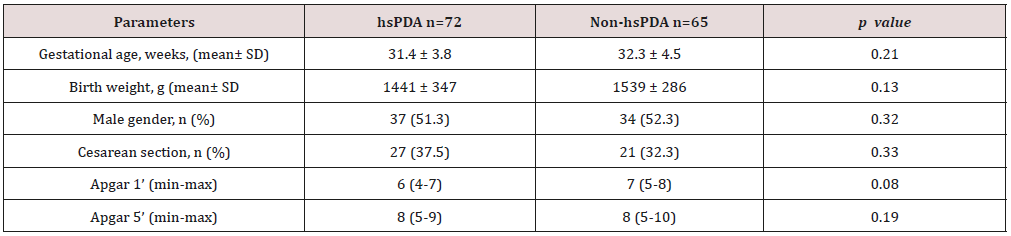
hsPDA: hemodynamically significant patent ductus arteriosus.
Table 2: Comparison of the platelet parameters of the study and control groups.
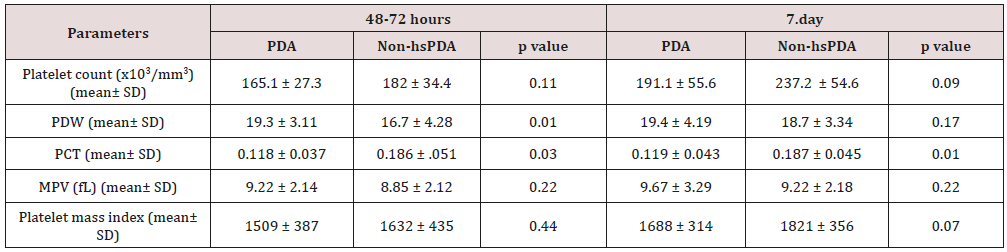
PDW: platelet distribution width; PCT: platocrit; MPV: mean platelet volume; Platelet mass index: the platelet count (103/mm3) X MPV (fL); hsPDA: hemodynamically significant patent ductus arteriosus.
Figure 1: Flowchart of study.

PPROM: Preterm premature rupture of the membranes; hsPDA: hemodynamically significant patent ductus arteriosus.
Discussion
Low oxygen pressure, elevated prostaglandin and nitric oxide levels are the main factors affecting continuity of the ductus arteriosus in the uterus. After birth, increased oxygen levels and decreased prostaglandin levels enable functional closure of the DA [13]. In addition to this mechanism, different mechanisms of closure of the ductus began to be discussed. The discussion began when Echtler et al. showed that platelets were attached to the lumen of the closed ductus arteriosus and confirmed this experimental finding via a retrospective study of preterm births [3]. After this animal study, various hypotheses about the role played by platelets in duct closure in newborns have been proposed. The most acceptable hypothesis is an effect of platelets on DA contraction, which occurs immediately after birth in term newborns, triggering hypoxia in the vessel wall by decreasing the blood flow in the venous lumen and vasa vasorum; in preterm newborns, the cells in the ductus wall are fed by the ductal lumen because of the absence of a vasa vasorum. As the ductus wall is thin, contraction is inadequate and endothelial damage and platelet aggregation thus develop because of vesselwall hypoxia. Based on this hypothesis, it was claimed that platelet counts were important in terms of DA closure in preterm infants, as they are in the pathophysiology of adult vascular diseases [14,15]. However, this hypothesis is not supported by the fact that platelet transfusion does not reduce the incidence of PDA in preterm newborns with immune thrombocytopenia and does not increase the PDA frequency in term newborns with severe thrombocytopenia secondary to Wiskott-Aldrich syndrome [16-20].
In Fujioka et al. [21-23]. the platelet count was not related to PDA diagnosis or treatment success. On the other hand, Echtler et al. [3,5,6] reported that a low platelet count increased the hsPDA incidence [24-25]. In some works performed after these contradictory studies, it was reported that large platelets create a greater potential risk of prothrombotic reactions; large platelets are more aggregated than small and normal platelets given the greater number of receptors such as thromboxane A2-B2 and glycoproteins IIb-IIIa on the surfaces of large platelets. It was suggested that the increased metabolic and enzymatic activities of dysfunctional thrombocytes, rather than the platelet count, were associated with PDA [26-29]. We sought to identify parameters related to platelet function associated with PDA. These remain controversial; all of MPV, PDW, PCT, and platelet mass index have been associated with cardiovascular diseases in adults [30-35]. In addition, in a limited number of studies on neonates, the MPV and PDW were shown to be associated with prematurity complications such as RDS and BPD [36-37].
In our study, no difference was found between the platelet counts of the hsPDA and control groups at 48-72 h and 7. day. In addition, there was no difference between the platelet counts of newborn who did and did not fail treatment. In conclusion, the platelet count was not a predictor of hsPDA diagnosis or treatment success. The results of our study contradict those of the two major meta-analyses conducted by Simon et al. and Mitra et al. but support the cohort study of Sallmon et al. [18-20]. PCT was lower and PDW was higher in the study groups than control groups and the difference between the two groups was statistically significant. However, MPV and platelet mass index were similar in both groups. Thus, we conclude that the PCT and PDW can be used to predict hsPDA but not treatment success. Demirel and Dizdar et al. [4]. reported that the PDW was higher in preterm infants with hsPDA than in control groups [38,39]. Bekmez et al [40]. reported that a low PCT increased the hsPDA incidence Demir et al.[41]. found a high MPV and a low platelet mass in the hsPDA group. We also excluded patients who received ibuprofen as ductus closure therapy because of potential effects on platelet count and functions. Infants born to mothers with prior pre-eclampsia, which affects platelet count and ductal flow because of the increased placental resistance, were also excluded [42-44]. We also excluded infants with perinatal asphyxia associated with an increased PDA, thrombocytopenia, and platelet dysfunction [45-47]. Newborns whose mothers had earlier received steroids were excluded because of possible effects on the platelet count. We thus excluded all pathologies that may affect platelet count and function and induce inflammation. There were some limitations of our study. The first limitation of our study is that it was retrospective in nature. The second limitation is modest sample size. As a result, according to our study, platelet count, MPV and platelet mass index cannot be used to predict either hsPDA or treatment success, but a low PCT and high PDW can be used predict hsPDA but not treatment success.
References
- Mirea L, Sankaran K, Seshia M, Ohlsson A, Allen AC, et al. (2012) Treatment of patent ductus arteriosus and neonatal mortality/ morbidities: Adjustment for treatment selection bias. J Pediatr 161(4): 689-694.e1.
- Sehgal A, McNamara PJ (2012) The ductus arteriosus: A refined approach. Semin Perinatol 36(2): 105-113.
- Echtler K, Stark K, Lorenz M, Kerstan S, Walch A, et al. (2010) Platelets contribute to postnatal occlusion of the ductus arteriosus. Nat Med 16(1): 75-82.
- Dizdar AE, Ozdemir R, Sari FN, Yurtutan S, Gokmen T, et al. (2012) Low platelet count is associated with ductus arteriosus patency in preterm newborns. Early Hum Dev 88(10): 813-816.
- Kulkarni VV, Dutta S, Sundaram V, Saini SS (2016) Preterm thrombocytopenia and delay of ductus arteriosus closure. Pediatrics 138(4): e20161627.
- Meinarde L, Hillman M, Rizzotti A, Basquiera AL, Tabares A, et al. (2016) C-reactive protein, platelets, and patent ductus arteriosus. Platelets 27(8): 821-823.
- Fujioka K, Morioka I, Miwa A, Morikawa S, Shibata A, et al. (2011) Does thrombocytopenia contribute to patent ductus arteriosus? Nat Med 17(1): 29-30.
- Bas-Suarez MP, Gonzalez-Luis GE, Saavedra P, Villamor E (2014) Platelet counts in the first seven days of life and patent ductus arteriosus in preterm very low-birthweight infants. Neonatology 106(3): 188-194.
- Murphy DP, Lee HC, Payton KS, Powers RJ (2016) Platelet count and associated morbidities in VLBW infants with pharmacologically treated patent ductus arteriosus. J Matern Fetal Neonatal Med 29(13): 2045- 2048.
- Brunner B, Hoeck M, Schermer E, Streif W, Kiechl-Kohlendorfer U, et al. (2013) Patent ductus arteriosus, low platelets, cyclooxygenase inhibitors, and intraventricular hemorrhage in very low birth weight preterm infants. J Pediatr 163(1):23-28.
- Shah NA, Hills NK, Waleh N, McCurnin D, Seidner S, et al. (2011) Relationship between circulating platelet counts and ductus arteriosus patency after indomethacin treatment. J Pediatr 158(6): 919-923.e1-2.
- Köksal N, Aygün C, Uras N (2018) Turkish Neonatal Society guideline on the management of patent ductus arteriosus in preterm infants. Turk Pediatri Ars 53(Suppl 1): 76-87.
- Hung YC, Yeh JL, Hsu JH (2018) Molecular Mechanisms for Regulating Postnatal Ductus Arteriosus Closure. Int J Mol Sci 19(7): E1861.
- Kasirer-Friede A, Kahn ML, Shattil SJ (2007) Platelet integrins and immunoreceptors. Immunol Rev 218: 247-264.
- Slomp J, Gittenberger-de Groot AC, Glukhova MA, Conny van Munsteren J, Kockx MM, et al. (1997) Differentiation, dedifferentiation, and apoptosis of smooth muscle cells during the development of the human ductus arteriosus. Arterioscler Thromb Vasc Biol 17(5): 1003-1009.
- Andrew M, Vegh P, Caco C, Kirpalani H, Jefferies A, et al. (1993) randomized, controlled trial of platelet transfusions in thrombocytopenic premature infants. J Pediatr 123(2): 285-291.
- Bussel JB, Zacharoulis S, Kramer K, McFarland JG, Pauliny J, et al. (2005) Clinical and diagnostic comparison of neonatal alloimmune thrombocytopenia to non-immune cases of thrombocytopenia. Pediatr Blood Cancer 45(2): 176-183.
- Simon S R, van Zogchel L, Bas-Suárez M P, Cavallaro G, Clyman R I, et al.2015Platelet counts and patent ductus arteriosus in preterm infants: A Systematic review and meta-analysis. Neonatology 108(2): 143-151.
- Mitra S, Chan AK, Paes BA (2017) Thrombosis and Hemostasis in Newborns (THIN) Group. The association of platelets with failed patent ductus arteriosus closure after a primary course of indomethacin or ibuprofen: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 30(2): 127-133
- Sallmon H, Weber SC, Huning B, Stein A, Horn PA, et al. (2012) Thrombocytopenia in the first 24 h after birth and incidence of patent ductus arteriosus. Pediatrics 130(3): e623-30.
- Fujioka K, Morioka I, Miwa A, Morikawa S, Shibata A, et al. (2011) Does thrombocytopenia contribute to patent ductus arteriosus?. Nat Med 17(1): 29-30.
- Bas-Suarez MP, Gonzalez-Luis GE, Saavedra P, Villamor E (2014) Platelet counts in the first seven days of life and patent ductus arteriosus in preterm very low-birthweight infants. Neonatology 106(3): 188-194.
- Murphy DP, Lee HC, Payton KS, Powers RJ (2016) Platelet count and associated morbidities in VLBW infants with pharmacologically treated patent ductus arteriosus. J Matern Fetal Neonatal Med 29(13): 2045- 2048.
- Kulkarni VV, Dutta S, Sundaram V, Saini SS (2016) Preterm thrombocytopenia and delay of ductus arteriosus closure. Pediatrics 138(4): e20161627.
- Meinarde L, Hillman M, Rizzotti A, Basquiera AL, Tabares A, et al. (2016) C-reactive protein, platelets, and patent ductus arteriosus. Platelets 27(8): 821-823.
- Gerday E, Baer VL, Lambert DK, Paul DA, Sola-Visner MC, et al. (2009) Testing platelet mass versus platelet count to guide platelet transfusions in the neonatal intensive care unit. Transfusion 49(10): 2034-2039.
- Kamath S, Blann AD, Lip GY (2001) Platelet activation: assessment and quantification. Eur Heart J. 22(17): 1561-1571.
- Zisk JL, Mackley A, Clearly G, Chang E, Christensen RD, et al. (2014) Transfusing neonates based on platelet count vs. platelet mass: A randomized feasibility-pilot study. Platelets 25(7): 513-516.
- Israels SJ, Rand ML, Michelson AD (2003) Neonatal platelet function. Semin Thromb Hemost 29(4): 363-372.
- Strauss T, Sidlik-Muskatel R, Kenet G (2011) Developmental hemostasis: primary hemostasis and evaluation of platelet function in neonates. Semin Fetal Neonatal Med 16(6): 301-304.
- Becchi C, Al Malyan M, Fabbri LP, Marsili M, Boddi V, et al. (2006) Mean platelet volume trend in sepsis: is it a useful parameter? Minerva Anestesiol 72(9): 749-756.
- Akpek M, Kaya MG, Yarlioglues M, Dogdu O, Ardic I, et al. (2011) Relationship between platelet indices and spontaneous echo contrast in patients with mitral stenosis. Eur J Echocardiogr 12(11): 865-870.
- Erdogan D, Tayyar S, Icli A, Uysal BA, Varol E, et al. (2012) Elevated mean platelet volume is associated with impaired coronary microvascular function in patients with idiopathic dilated cardiomyopathy. Platelets 23(3): 177-183.
- O Malley T, Langhorne P, Elton RA, Stewart C (1995) Platelet size in stroke patients. Stroke 26(6): 995-999.
- Varol E, Uysal BA, Ozaydin M (2011) Platelet indices in patients with pulmonary arterial hypertension. Clin Appl Thromb Hemost 17(6): E171-174.
- Canpolat FE, Yurdakok M, Armangil D, Yiğit S (2009) Mean platelet volume in neonatal respiratory distress syndrome. Pediatr Int 51(2): 314-316.
- Dani C, Poggi C, Barp J, Berti E, Fontanelli G, et al. (2011) Mean platelet volume and risk of bronchopulmonary dysplasia and intraventricular hemorrhage in extremely preterm infants. Am J Perinatol 28(7): 551- 556.
- Demirel G, Yilmaz A, Vatansever B, Tastekin A (2018) Is high platelet distribution width in the first hours of life can predict hemodynamically significant patent ductus arteriosus in preterm newborns?. The Journal of Maternal-Fetal & Neonatal Medicine.
- Dizdar AE, Ozdemir R, Sari FN, Yurtutan S, Gokmen T, et al. (2012) Low platelet count is associated with ductus arteriosus patency in preterm newborns. Early Hum Dev 88(10): 813-816.
- Bekmez BO, Tayman C, Buyuktiryaki M, Cetinkaya AK, Cakır U, et al. (2018) A promising, novel index in the diagnosis and follow-up of patent ductus arteriosus: Red cell distribution width-to-platelet ratio. J Clin Lab Anal 32(9): e22616.
- Demir N, Peker E, Ece I, Agengin K, Bulan KA, et al. (2016) Is platelet mass a more significant indicator than platelet count of closure of patent ductus arteriosus? J Matern Fetal Neonatal Med 29(12): 1915-1918.
- Sola-Visner M, Sallmon H, Brown R (2009) New insights into the mechanisms of nonimmune thrombocytopenia in neonates. Semin Perinatol 33(1): 43-51.
- Sallmon H, Sola Visner M (2012) Clinical and research issues in neonatal anemia and thrombocytopenia. Curr Opin Pediatr 24(1): 16-22.
- Itabashi K, Ohno T, Nishida H (2003) Indomethacin responsiveness of patent ductus arteriosus and renal abnormalities in preterm infants treated with indomethacin. J Pediatr 143(2): 203-207.
- Nadkarni J, Patne SK, Kispotta R (2012) Hypoxia as a predisposing factor for the development of early onset neonatal thrombocytopenia. J Clin 1(3): 131-134.
- Christensen RD, Baer VL, Yaish HM (2015) Thrombocytopenia in late preterm and term neonates after perinatal asphyxia. Transfusion 55(1): 187-196.
- Herdy GV, Lopes VG, Aragão ML, Pinto CA, Tavares, et al. (1998) Perinatal asphyxia and heart problems [in Portugese]. Arq Bras Cardiol 71(2): 121-126.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...


