Lupine Publishers Group
Lupine Publishers
Menu
Case Report(ISSN: 2770-5447) 
Ortner’s Syndrome: A Giant Pulmonary Artery Aneurysm, A Rare Etiology Volume 3 - Issue 4
Sanjeev Asotra*, Rahul Nijhawan, Rajesh Sharma, Neeraj Ganju and Shivani Rao
- Department of Cardiology, I.G.M.C Shimla, H.P India
Received:May 24, 2021; Published: June 9, 2021
Corresponding author: Sanjeev Asotra, Department of Cardiology, I.G.M.C Shimla, H.P India
DOI: 10.32474/ACR.2021.03.000169
Abstract
Ortner’s syndrome or Cardiovocal syndrome is hoarseness due to left recurrent laryngeal nerve palsy caused by mechanical affection of nerve from enlarged cardiovascular structures. A middle-aged female presented to us with a long history of hoarseness of voice and exertional dyspnea. The diagnosis of pulmonary artery aneurysm presenting as Ortner’s syndrome was made.
Keywords:Ortner’s; vocal cord palsy; Cardiovocal; aneurysm; pulmonary artery; PAA; PAH
Introduction
Hoarseness is a common symptom in clinical practice, and a variety of local laryngeal and extra laryngeal conditions are implicated in hoarseness. The most common extra laryngeal cause of left vocal cord palsy is bronchogenic carcinoma. Ortner’s syndrome is hoarseness due to recurrent laryngeal nerve involvement in cardiovascular disease. We present a case of pulmonary artery aneurysm causing left vocal cord palsy. The management of these patients is challenging and there are no ideal recommendations.
Case Report
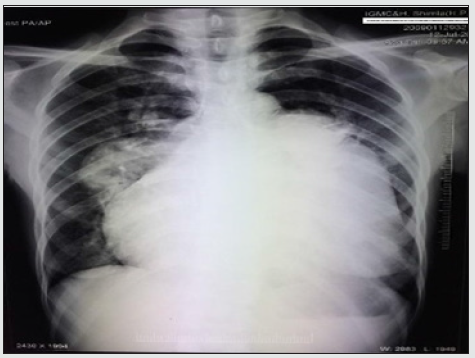
A 49-year-old female presented with chief complaints of exertional shortness of breath and hoarseness of voice for the last 2 years. She denied any history of tuberculosis, chronic cough, DVT, or smoking. Clinical examination revealed a thin built patient with BP- 100/60 mmHg, pulse rate (PR)-96 bpm regular, oxygen saturation- 89% in all limbs at room air. Her jugular venous pressure revealed prominent a wave and c-v descent. Cardiac examination revealed normal first and narrow split second heart sound with a loud pulmonary component, long early diastolic murmur in the pulmonary area starting with P2 with pansystolic murmur grade 3/6 in tricuspid area. An indirect laryngoscopic exam revealed left true vocal cord palsy. An electrocardiogram (figure 1) revealed regular rhythm with a heart rate of 80 bpm, right axis deviation, right atrial enlargement, prolonged PR interval with right ventricular hypertrophy. Chest x-ray (Figure 2) showed cardiomegaly with RVtype apex, right atrial enlargement, dilated main pulmonary artery with prominent pulmonary conus, peripheral pruning, and normal lung parenchyma.
Figure 1: ECG at presentation showing regular rhythm, right axis deviation, P-pulmonale, prominent R wave in V1, and right ventricular hypertrophy.
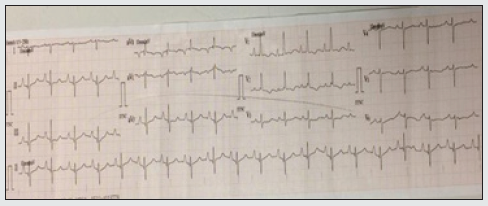
Figure 2: CXR PA view showing cardiomegaly, right atrial enlargement with dilated MPA, RPA, and LPA.
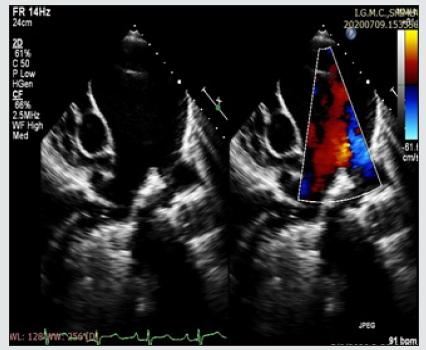
2D-echocardiography (Figure 3) revealed dilated right atrium and Dysfunctional right ventricle. The patient had severe PAH with moderate tricuspid regurgitation with a gradient of 103 mmHg, severe pulmonary regurgitation, and aneurysmally dilated main pulmonary artery. TAPSE was 15.LV systolic function was normal. There was evidence of mild pericardial effusion. Contrast echo did not reveal any shunt lesion.
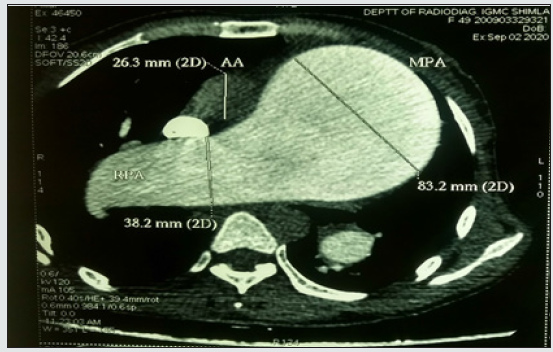
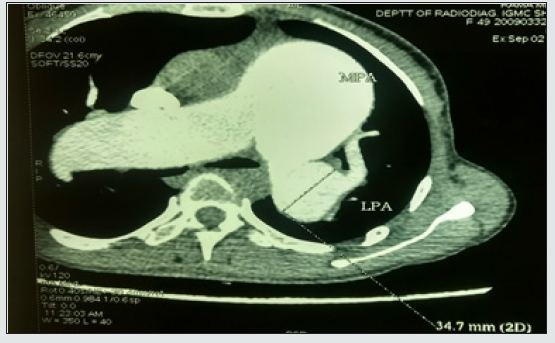
Computed tomography of the thorax (Figures 4 and 5) showed aneurysmally dilated main pulmonary artery (MPA), right pulmonary artery (RPA), and left pulmonary artery (LPA) with signs of pulmonary arterial hypertension (PAH) and cardiomegaly. MPA, RPA, and LPA measured 8.32 cm,3.82 cm, and 3.47 cm respectively. Lab investigations were normal, viral markers and ANA profile were negative. Arterial blood gas revealed an oxygen saturation of 91% with mild hypoxemia. Her pulmonary function test was normal.
The clinical symptoms in combination with imaging findings were consistent with a giant pulmonary artery aneurysm with severe PAH causing cardiovocal syndrome. The patient refused cardiac catheterization and was discharged with medical treatment and remains under regular follow-up.
Discussion
Cardiovocal syndrome was originally described in 1897 by Nobert Ortner in three patients with severe mitral stenosis caused by compression of the left recurrent laryngeal nerve by the enlarged left atrium[1,2]. Mitral valve prolapse, thoracic aortic aneurysm, aortic dissection, cor pulmonale, and pulmonary artery dilatation secondary to increased pulmonary artery pressure with any reason have also been described in the etiology of Ortner’s syndrome. The pathophysiological mechanism in PAH with pulmonary artery dilatation is thought to be due to compression of the left recurrent laryngeal nerve between the aorta and dilated pulmonary artery. Because of the anatomical course of the recurrent laryngeal nerves, the left side is more vulnerable and it is 1.75 times more frequently affected than the right [3-7]. Pulmonary artery (PA) aneurysms (PAAs) are rare and infrequently diagnosed. Brown and Plotnick defined PAA as a PA with a diameter exceeding 40 mm[8]. Deterling and Clagett discovered only 8 cases of PAAs in 109571 consecutive postmortem examinations [5]. PAAs generally occurred in a younger age group than aortic aneurysms with an equal sex incidence[6]. Eighty-nine percent of all PAAs were located in the main PA, whereas only 11% were located in the pulmonary branches[7]. When affecting the PA branches, PAAs in the left PA were more common than in the right PA[5] .
Depending on the underlying condition responsible for PAA formation, patients frequently present with right atrial and ventricular hypertrophy, right heart failure, tricuspid regurgitation caused by annulus dilatation, and mild pericardial and pleural effusion[9].
A common complication of PAA includes in situ thrombosis, pulmonary artery dissection, compression of adjoining structures like left main, adjoining bronchus, and recurrent laryngeal nerve like in our case. PAA can progress to rupture and can present as it resulting in lethal hemoptysis, asphyxiation, and sudden death and is the most common cause of mortality[10]. The treatment of PAH with vasodilators does not affect the growth rate of PAA. Surgery remains the mainstay of treatment in PAA. Surgery indications include absolute PAA diameter ≥5.5 cm, an increase in the diameter of the aneurysm of ≥0.5 cm in 6 months, compression of adjacent structures, thrombus formation in the aneurysm sac, appearance of clinical symptoms, evidence of valvular pathologies or shunt flow, verification of PAH and any signs of rupture or dissection. A lung transplant may be an option in the presence of significant parenchymal or progressive pulmonary vascular disease. [9][11, 12]. PAA due to PAH presenting as cardiovocal syndrome is rare. No clear guidelines are available in the management of this disease. Further registry data are needed on the progression and natural history of pulmonary artery aneurysms.
References
- Ortner N (1972) “Recurrenssl¨ahmung bei mitral stenose”. Wien Klein Wochenschr10: 753–755.
- Solanki SV, Yajnik VH. Ortner’s Syndrome. Indian Heart J 24(1): 43-46.
- Paquette CM, Manos DC, Psooy BJ (2012) “Unilateral vocal cord paralysis: a review of CT findings, mediastinal causes, and the course of the recurrent laryngeal nerves. Radiographics 32(3): 721-740.
- Subramaniam V, Herle A, Mohammed N, Tahir M (2011) Ortner’s syndrome: case series and literature review. Braz J Oto¬rhinolaryngol 77: 559-562.
- Deterling RA Jr, Clagett OT (1947) Aneurysm of the pulmonary artery: a review of the literature and report of a case. Am Heart J 34(4): 471-499.
- Blades B, Ford W, Clark P (1950) Pulmonary artery aneurysms: report of a case treated by surgical intervention. Circulation 2(4): 565-571.
- Metras D, Ouattara K, Quezzin-Coulibaly A (1987) Aneurysm of the pulmonary artery with cystic medial necrosis and massive pulmonary valvular insufficiency: report of two successful surgical cases. Eur J Cardiothorac Surg 1(2): 119-124.
- Brown JR, Plotnick G (2008) Pulmonary artery aneurysm as a cause for chest pain in a patient with Noonan’s syndrome: a case report.Cardiol 110(4): 249-251.
- Kreibich M, Siepe M, Kroll J, Hohn R, Grohmann J, et al. (2015) Aneurysms of the pulmonary artery. Circulation 131(3): 310-316.
- Butto F, Lucas jr RV, Edwards JE (1987) Pulmonary artery aneurysm Chest 91(2): 237-241.
- Mulpuru SK, Vasvada BC, Punukollu GK, Patel AG (2008) Cardiovocal syndrome: A systematic review. Heart, Lung, and circulation 17(1): 1-4.
- Senabaklavaci O, Kanieko Y, Bartunek A, Brunner C, Kurkciyan E, et al. (2001) Rupture and dissection in pulmonary artery aneurysm: Incidence, cause, and treatment-Review and case report. J thorac cardiovasc Surg 121(5): 1006-1008.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...