
Lupine Publishers Group
Lupine Publishers
Menu
Research Article(ISSN: 2770-5447)
Discovery and Development of an Oral, Small Molecule PCSK9/LDLR Antagonist Volume 3 - Issue 4
Nabil A. Elshourbagy1*, Harold Meyers1, Sherin S Abdel-Meguid1, Kavitha Godugu2, Taher A Salaheldin2, Dhruba J Bharali2 and Shaker A Mousa2
- 1Shifa Biomedical Corporation, 1 Great Valley Parkway, Suite 8, Malvern, PA 19355, USA
- 2The Pharmaceutical Research Institute, Albany College of Pharmacy and Health Sciences, 1 Discovery Drive, Rensselaer, NY 12144, USA
Received:April 28, 2021; Published: May 5, 2021
Corresponding author: Nabil A. Elshourbagy, Shifa Biomedical Corporation, 1 Great Valley Parkway, Suite 8, Malvern, PA 19355, USA
DOI: 10.32474/ACR.2021.03.000167
Abstract
PCSK9 is a well validated target for the treatment of hypercholesterolemia. Several pharmaceutical companies focused on the development of injectable monoclonal antibodies. Shifa Biomedical has focused on developing orally bioavailable small molecule PCSK9/LDLR antagonists. PCSK9 antagonists were identified through virtual screening at the PCSK9 surface where the LDLR-EGF-A binds. An SAR study of many analogues were done for their ability to antagonize the PCSK9/LDLR interaction in an in vitro ELISA assay, upregulate the LDLR in a cell-based assay, and increase LDL uptake in a fluorescent Dil-LDL assay. The most potent compound meeting these criteria was P-4, formulated by a nanocrystal/targeted delivery approach for the in vivo studies. Nano formulated P-4 was named P-21. P-4 exhibited a concentration-dependent inhibition of the PCSK9/LDLR interaction with an IC50 in the nanomolar range, an increase in the level of LDLR in recombinant cell-based assays, and a significant increase in the fluorescently labeled DiILDL uptake in the nanomolar range. P-4 also exhibited a good safety profile as demonstrated in vitro and in in vivo animal models. P-21 showed improved PK and PD of P-4. Oral administration of P-21 at 1, 3, 10, and 30 mg/kg in C57BL/6 mice fed a high-fat diet resulted in approximately a 20, 40, 60, and 90% LDL-C lowering, respectively. The identification of a small molecule orally bioavailable antagonist against PCSK9 could lead to a new class of therapies to treat hypercholesterolemia, especially for those patients that are statin resistant or stain intolerant.
Keywords: Atherosclerosis; low-density lipoprotein cholesterol; PCSK9 small molecule inhibitors; hypercholesterolemia; cholesterol
Abbreviations: AHA: American Heart Association; CETP: cholesteryl ester transfer protein; DHA: docosahexaenoic acid; DLS: dynamic light scattering; EC50: effective concentration ; EPA: eicosatetraenoic acid; ELS: electrophoretic light scattering; HPMC-AS: Hydroxypropyl methylcellulose acetate succinate; LDL-C: low-density lipoprotein-cholesterol; LDLR-EGFA-A: low-density lipoprotein receptor epidermal growth factor homology domain A; LDLR: low-density lipoprotein receptor; PCSK9: proprotein convertase subtilisin-like kexin type 9; PEG: polyethylene glycol; PLGA: poly-(lactic-co-glycolic acid) PK: pharmacokinetics; PD: pharmacodynamics ; SLN: solid lipid nanoparticle
Introduction
Dyslipidemia contributes to the development of atherosclerosis, an inflammatory condition resulting from multiple and cumulative risk factors, each of which contributes in varying ways to the development and severity of the condition [1]. The risk of atherosclerosis and heart attacks is strongly correlated to blood cholesterol levels [2]. According to the American Heart Association (AHA), atherosclerosis, partly because of dyslipidemia, is the leading cause of cardiovascular diseases [2]. The discovery of proprotein convertase subtilisin-like kexin type 9 (PCSK9) has emerged as a major breakthrough in developing new cholesterol-lowering drugs [3-5].
The link between PCSK9 and cholesterol metabolism was established by discovering that selected mutations in the PCSK9 gene caused autosomal dominant hypercholesterolemia, [3] suggesting that the mutations confer a gain-of-function [6]. Conversely, three loss-of-function mutations in PCSK9 (Y142X, L253F, and C679X) were identified in African Americans [7]. These mutations reduce low-density lipoprotein- cholesterol (LDL-C) levels by 28-88% [7]. It is now well established that modulation of the low-density lipoprotein receptor (LDLR) degradation pathway is mediated by PCSK9 [8]. Upon LDL binding to LDLR, the LDLR/LDL complex is endocytosed, LDL is released in the acidic environment of the endosome where it undergoes lysosomal degradation [9] and the LDLR is recycled to the cell surface. Each LDLR molecule undergoes this process multiple times [9]. Conversely, secreted PCSK9 binding to the LDLR on the cell surface causes degradation of the LDLR and prevents its recycling [9]. Hence, inhibiting this PCSK9/LDLR interaction now represents a proven therapeutic modality to lower LDL-C. Although statins (HMG CoA reductase inhibitors) significantly reduce cardiovascular- related morbidity and mortality in patients with and without coronary artery disease, there are some people who are statin intolerant through muscle myopathy and some people who are statin resistant. Although Zetia (ezetimibe) inhibits the intestinal absorption of cholesterol, it only lowers cholesterol levels by 15-20% in most patients [10]. While statins are effective in lowering LDL-C, it is estimated that 15-20 million cardiovascular patients do not attain the goal of 70 mg/dL LDL-C (so-called ‘statin resistant’) [11], 4 million patients are statin intolerant and discontinue statin use due to severe side effects [12], and 1.3 million patients are familial hypercholesterolemic (1 out of 250 worldwide) [13]. These and other patients will dramatically benefit from an aggressive treatment of hypercholesterolemia.
To our knowledge, no oral small molecular antagonist of PCSK9 has yet to enter clinical trials. As of today, few companies have sustained the development of PCSK9 inhibitors. Sanofi and Amgen have successfully launched injectable mAb therapies against PCSK9. [14,15] In 2015, alirocumab (Praluent) from Sanofi/Regeneron and evolocumab (Repatha) from Amgen were FDA approved and marketed [14,15]. The Medicines Company/Alnylam have an siRNA molecule, Inclisiran, that inhibits PCSK9 production and they filed an NDA in December 2019 (Novartis recently acquired the asset for almost $10 billion) [16]. Among the non-statin cholesterol lowering drugs, Esperion Therapeutics has developed bempedoic acid, with ~20% LDL lowering. The company’s second product is a combination therapy of bempedoic acid and Zetia, with 40- 50% LDL- C lowering [17]. Both drugs were recently approved for marketing by the FDA.
Because of the high costs of the two marketed injectable PCSK9 mAbs (~$14,000 per year), Sanofi and Amgen have reduced the cost in order to secure broader insurance coverage and lower outof- pocket costs for patients [18]. We have been developing a small molecule, orally bioavailable PCSK9 antagonist that interferes with the LDLR-EGF-A (Epidermal Growth Factor precursor homology domain A) domain binding at the PCSK9 surface. The availability of an oral small molecule inhibitor of PCSK9 with good efficacy and anticipated tolerability/safety would represent a tremendous advance and opportunity. In this regard, it is important to note that oral versus injectable therapeutics have historically dominated the traditional drug discovery market because they are cost effective, easily administered, and preferred by patients, doctors, and payers alike [19]. Here we present our innovative work in which a hepatic-targeted formulation approach was applied and optimized to enhance the oral bioavailability, pharmacokinetics (PK) and pharmacodynamics (PD) profile of our lead compound.
Methods
Lead Identification
Shifa has successfully combined a robust virtual screening method with validated cell- based recombinant assays to screen for and identify inhibitors of the PCSK9/LDLR interaction. We targeted the site on PCSK9 where it binds to the LDLR. This site has been defined at the atomic level by crystallographic studies [5].
a. Virtual screening: Protein in structure preparation, docking, and compound acquisition: The crystallographic atomic coordinates of the PCSK9/LDLR-EGF-A complex were available in the Protein Data Bank (PDB ID: 3BPS) [5]. We excised from the structure the LDLR EGF-A domain, the PCSK9 prodomain and V domain, and all water molecules. The remaining catalytic domain was used for docking, and compounds were docked at the PCSK9 surface where the LDLR (EGF-A domain) binds. The region for docking was defined by the center of mass of the residue Phe379 of the PCSK9 chain. We screened more than 1,000,000 diverse, “drug-like” compounds, selected from over 8 million purchasable molecules at the time in the ZINC drug-like database (http://zinc.docking.org/). In addition, we screened approximately 410,000 compounds from the ZINC all-purchasable database that have a MW > 500 Daltons to include larger molecules potentially better capable of disrupting the protein-protein interaction between PCSK9 and its receptor. Furthermore, we docked a database of hundreds of molecular fragments. After visual inspection of several hundred of the top-scoring compounds docked on the PCSK9 catalytic domain surface where the LDLR interacts (EGF-A domain) for each database, we selected and purchased 479 screening compounds using medicinal chemistry criteria and other considerations, including emphasis on selecting a diverse set of chemotypes, vendor quality, and compound costs. All compounds were tested in our PCSK9/LDLR assays for their ability to upregulate the LDLR.
b. In vitro binding: An in vitro ELISA assay kit was used (Circulex). For screening inhibitors of the PCSK9/LDLR interaction, different concentrations (0.01 μM to 100 μM) of selected compounds were incubated with His-tagged PCSK9 and then added to wells that were pre-coated with recombinant LDLR-AB domain. After incubation, the plate was washed, and the amount of recombinant His-tagged PCSK9 was measured using the biotinylated anti- His-tag and horseradish peroxidase conjugated streptavidin and quantitated using a BioTek Synergy-2 plate reader. The effect of each compound on the PCSK9 binding to the recombinant LDLR-AB domain was calculated.
c. PCSK9 synthesis, processing, and secretion: This assay was established to ensure that any validated hits from the in vitro binding assay (vide supra) did not have an effect on PCSK9 synthesis, processing, or secretion. HEK-293 cells (ATCC) were seeded into 96-well plates in DMEM containing 10% Fetal Bovine Serum media and incubated overnight at 37ºC. Cells were transiently transfected with pcDNA3.1/human full length PCSK9 cDNA construct using the lipofectamine- LTX (Thermo Fisher). Compounds (25 μM) or vehicle were added, followed by an additional 43 hr of incubation. Cellular PCSK9 and secreted PCSK9 were analyzed using western blot analysis, imaged, and quantitated using a LAS-4000 (GE).
d. Upregulation of LDLR by PCSK9 inhibitors: This assay was used to demonstrate that expression of PCSK9 in HEK-293 cells resulted in increased degradation of the LDLR. pcDNA3.1/ human PCSK9 and the pcDNA3.1/human LDLR constructs were used to transfect mammalian cells. HEK-293 cells were seeded (1 × 105 cells/well) in 24-well plates. Expression plasmids (1 μg/well) were transiently transfected into HEK-293 cells with lipofectamine (Thermo Fisher). Twenty-four to 48 hr after transfection, cells were lysed. Cell lysate and media were subjected to SDS-PAGE and immunoblot analysis using an anti-PCSK9 or LDLR antibody.
e. Uptake of fluorescent Dil-LDL: HepG2 cells (ATCC) were plated in 96-well plates and allowed to grow overnight. Cells were transiently transfected with human PCSK9 cDNA constructs using the lipofectamine-LTX as described above and incubated overnight. Compounds (1.2 μM) were added to the cells followed by the addition of fluorescent Dil-LDL. Cells were washed extensively, and the fluorescent Dil-LDL taken up by the cells was measured using the Synergy-2 plate reader.
f. Cell viability: All compounds that upregulated the endogenously expressed LDLR were used to test for in situ cell viability. HEK-293T cells or HepG2 cells were seeded in 96-well plates in a cell media containing 10% Fetal Bovine Serum and incubated overnight at 37ºC. Compounds (25 μM) were added to cells after 24 hr and incubated for an additional 48 hr. Cell viability was assayed using Resazurin (Sigma 199303) and a Synergy-2 plate reader. Compounds that showed cell toxicity (>100 μM) were excluded.
Formulation Studies
Using the above strategy coupled with several rounds of lead optimization on multiple validated hits representing several chemotypes, we identified P-4 as our best drug substance (see Results below), and it was used to develop and optimize our lead product; the nanoformulated P-4 (called P-21).
a. Liver-targeted nanoformulation studies: Highly lipophilic drugs or poorly water- soluble ones such as P-4 belong to class IV pharmaceuticals. Our goal was to utilize different nanoformulations for P-4 to increase its solubility and permeability for improved oral delivery. The use of surfactants to improve the dissolution performance of poorly soluble drug products is probably the most basic and oldest known method. Surfactants reduce surface tension and improve the dissolution of lipophilic drugs in aqueous medium as described below. Hydroxypropyl methylcellulose acetate succinate (HPMC-AS), a natural polymer, was used as a surfactant for nano formulation of P-4. HPMC-AS is a mixed ester of HPMC acetate and succinic acid and is FDA-approved for enteric drug coatings [20]. As an enteric coating material, it has good film-forming properties without plasticizer and good solubility in the upper part of the small intestine (duodenum) and thus can increase P-4’s absorption in the small intestine. The dispersions were prepared as follows: P-4 was solubilized in HPMC-AS using sonication for 10 min. For liver-targeting, Alginic acid was used, which is a natural polysaccharide refined from brown seaweeds that has remarkable targeting affinity to the liver resulting in accumulation of drug in the liver [21]. After additional optimization of the nano formulation and based on a variety of factors including particle size, the nano- encapsulation method was the best formulation obtained [22]. The preparation procedure of P-21 was as follows: 69 mg of P-4, 69 mg of PVP (average MW 40,000), 100 mg of HPMC-AS and 1 mg of Alginic acid in 6 mL of DMSO were added slowly to 60 mL of water under probe sonication and then stirred for 30 min at room temperature, forming P-21 as a liver-targeted nano-encapsulated suspension. To remove any unformulated substrates, the P-21 nano-suspension was washed twice with 1% PVA using centrifugation (14,800 x g, 4°C, 30 min). The obtained P-21 was re-dispersed in 10 mL of 1% PVA to undergo physicochemical characterization before using in subsequent PK and PD studies.
b. Characterization of P-21: Size distribution and zeta potential of P-21 in aqueous dispersions were measured with Dynamic Light Scattering (DLS) and Electrophoretic Light Scattering (ELS) techniques using a Malvern zeta sizer (Malvern Instrumentation Co.). Fifty mg of the lyophilized nanoparticles were re- suspended in 2 mL of water. This solution was placed into a 3 mL, 4-sided, clear plastic cuvette and measured directly. Topographic imaging was captured using a High-Resolution Transmission Electron Microscope (HRTEM) where 20 μL of diluted P-21 was placed on a 300-mesh carbon coated grid, then coated with 1% phosphotungstic acid and left to dry for 15 min before inserting the platinum sample holder for TEM imaging under 200 KV. HPLC was used for P-4 quantification in the final P-21 nano formulation. Encapsulation efficiency of the P-21 nano formulation was determined by analyzing the P-4 loading in the P-21 compared to the amount of P-4 initially added. After lyophilization, the weighed P- 21 powder was dispersed in 3 mL of DMSO for 30 min. The amount of P-4 in the DMSO was determined at 337 nm using HPLC and a calibration curve. Entrapment efficiency (%) and P-4 loading ratio (%) were calculated according to Sudha et al.[23].
In Vivo Pharmacology
Animal studies for PK and PD were carried out under an IACUC approved protocol at the VA animal facility (Albany, NY).
a. PK analysis: Male C57BL/6 mice (purchased from Taconic, Rensselaer, NY), 4-5 weeks old, were housed 5/cage in a room maintained at 20 ± 2°C with a humidity of 50 ± 10% and a 12 hr light/dark cycle. The animals were fed a standard pelleted mouse chow. Single IV (10 mg/kg) and oral (30 mg/kg) doses of P-4 formulations were administered and 50 μL of blood samples were collected using anti- coagulated capillary tubes at 0.25, 0.5, 1, 3, 6, 12, 24, and 48 hr post- administration for PK profiles using an established LC/MS/MS method.
b. PD analysis using a nutritionally induced hypercholesterolemia mouse model: Male C57BL/6 mice were housed 4/cage under climate-controlled conditions as described above. Mice were fed a high-fat diet (TD.06414, Harlan Research Diet Inc.) that provides 60% of calories from fat sources to increase total cholesterol. The nutritionally induced mouse is therefore a suitable model for examining the effects of P-4 formulations in lowering LDL-C levels. Male C57BL/6 mice were fed either a commercial chow diet (Prolab RMH 3000, PMI feeds) to serve as a negative control, or the high-fat diet. Plasma was collected once weekly to monitor the level of LDL-C and PCSK9 levels. After 4 weeks of feeding on the high-fat diet, mice were randomly assigned to one of 6 different groups of 6 mice each such that the average of each biomarker level was comparable among the different groups. One group was treated with vehicle (Phosphate-buffered saline; PBS), and the other groups were treated orally with P-21 at 1, 3, 10, and 30 mg/kg or subcutaneously at 3 mg/kg. After 24 hr, 1 week, and 2 weeks of treatment, blood samples (75 μL) were collected from the retro-orbital venous plexus via heparinized capillary tubes containing 2 USP units of ammonium heparin per tube (Carolina). Plasma was separated immediately by centrifugation (5,000 x g for 5 min) at room temperature and then kept at -80°C until assayed for lipid profile. Plasma total and free cholesterol, cholesterol esters, LDL-C, HDL-C, and PCSK9 levels were measured.
Statistics
Statistical analysis was done using GraphPad Prism. All data are presented as mean ± standard error of the mean, and statistical significance was defined as *P <0.01, **P<0.001, ***P <0.0001.
Results
Lead Identification and Optimization
We tested almost 500 compounds for their potential to upregulate the LDLR in HEK- 293 cells transfected with LDLR/ PCSK9 using 50 μM of the compounds. Each compound was added to the cell media in triplicate and intracellular LDLR was detected as described in Methods. From the initial screening, 12 compounds from our virtual screen were identified as potential hits. Hits confirmation was repeated in triplicate using a range of 25-0.1 μM of compounds. Compounds that showed cell toxicity were excluded. Five of the best compounds were confirmed to upregulate LDLR in a concentration-dependent manner in HEK-293T transfected cells as compared to control. These hits exhibited low micromolar or better effective concentrations (EC50 <5 μM) and represent pyrrazole analogs, N-aryl(heteroaryl) thioacetamides, and N-aryl(heteroaryl) amides. None of the confirmed hits had any measurable effect on PCSK9 synthesis, processing, or secretion (data not shown).
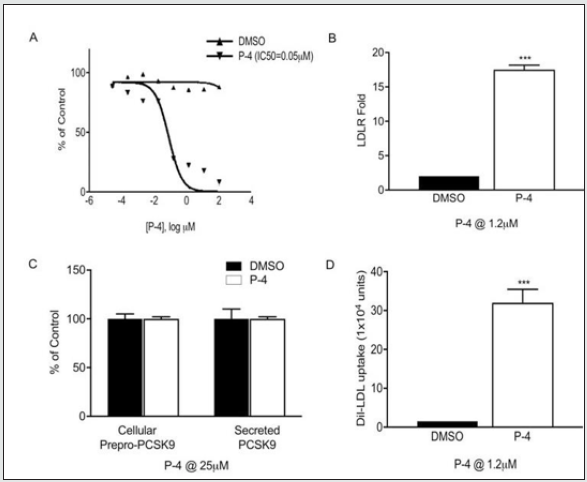
We initiated lead optimization studies by developing analogs for structure activity relationship (SAR) studies of the pyrrazole, N-aryl(heteroaryl) thioacetamides, and N- aryl(heteroaryl)amide hits. Analogs from each class were identified and demonstrated an LDLR fold increase between 8- to 13-fold at 25 μM in HEK-293 cells (data not shown). We obtained a number of additional analogs from substructural or similarity searches in vendor databases around our lead compounds, including 75 pyrrazole analogs, 9 analogs of an N-aryl-1-H indole-2-carboxamide, and 54 analogs of the closely related N-aryl benzofuran-2-carboxamides. From the screening, 20 compounds were selected for further validation using various concentrations of each compound ranging from 0.01 μM to 100 μM. After testing these compounds in our in vitro PCSK9/LDLR binding assay, 5 of the latter compounds were identified as our best hits in the low micromolar range. Based on these observations, several rounds of design, synthesis, and testing of a new library of benzofuran-2-carboxyanilides (over 35 compounds) in both in vitro and cell- based assays resulted in the identification of 4 lead compounds. The best compound, P-4 (drug substance, P-4) was selected for in vivo efficacy studies because it had the following properties (Figure 1):
1. LDLR/PCSK9 Binding (Figure 1A): The best compounds were selected from our binding assay for their potential to inhibit the PCSK9/LDLR interaction using different compound concentrations. The most potent benzofuran compound, P-4, had an IC50 of 50 nM (i.e., solubility optimized by adding 10% DMSO) and was selected for further evaluation in multiple cellbased assays.
2. Effect on LDLR Upregulation in HEK-293 Cells (Figure 1B): Transfecting the cells expressing LDLR with a decreasing concentration of PCSK9 cDNA resulted in a decrease in the degradation of the LDLR. Cells transfected with PCSK9 expressed both the unprocessed (78K) and the processed PCSK9 (64K) (cells), and processed PCSK9 (media). Cells that were transfected with LDLR showed expression of the LDLR in the cells. However, cells that were transfected with both LDLR and PCSK9 resulted in the disappearance of the LDLR in the cell, which indicates that the PCSK9 expression results in the degradation of the LDLR (data not shown). We tested our compounds for their ability to reduce the degradation of the LDLR. Figure 1B shows that P-4 exhibited a significant increase in the LDLR upregulation (>16-fold).
3. Effect on Synthesis, Processing, and Secretion of PCSK9 (Figure 1C): To eliminate the possibility that these compounds interfere with PCSK9 synthesis, processing, or secretion, we tested their effect as described. The results show that P-4 exhibited no effect on the synthesis, processing, or secretion of PCSK9 either in the cells or in the media.
4. Uptake of Dil-LDL in HepG2 Cells In Situ (Figure 1D): To confirm that these PCSK9 antagonists upregulate functional LDLR, we tested the ability of our lead compounds to enhance the uptake of fluorescent Dil-LDL in HepG2 cells. As shown in Figure 1D, P-4 exhibited significant increases in the Dil-LDL uptake at 1.2 μM and was selected for further analysis in the in vivo studies.
Figure 1: (A) In vitro binding of P-4 using an ELISA assay kit, (B) effect of P-4 on LDLR upregulation of LDLR in HEK-293 cells expressing PCSK9 and LDLR, (C) effect of P-4 on PCSK9 synthesis, processing, and secretion, (D) effect of P-4 on the uptake of fluorescent Dil-LDL in HepG2 cells. Error bars represent mean ± standard error of the mean, ***P <0.0001.
Nanoformulation for Liver-Targeted Delivery
Formulation strategies in early-stage drug development are critical, so we focused our strategy on applying the best formulation early in our drug development process to avoid costly latestage failures. We tested the effect of many soluble formulations and nano formulations on the PK of P-4 and its oral bioavailability. Many conventional formulations with P-4 were attempted with acceptable PK (>20% bioavailability), however, we were unable to obtain more than 20% LDL-C lowering. In order to improve the oral delivery and oral bioavailability, we utilized various nanocrystal/ nano formulations for P-4. These initially included nanosuspension [24], micellar solubilization [25], and chemical modification methods [26].
All the formulations were tested for efficacy but none of them improved the efficacy of P-4 (data not shown). Therefore, we tried using nanotechnology for targeted delivery. Below is the description of our best nano formulation for liver- targeted delivery. The average particle size of the P-21 nanoformulation of P-4 is 132.5 nm and the zeta potential is -3.53 mV. The TEM image showed hexagonal shaped clusters filled with P-21 clusters of hydroxyethyl cellulose acetate succinate. These capsules contained many nanosized spherical-shaped clusters, 50-200 nm diameter, each filled with P-4. The encapsulation efficiency of P-21 nano formulation was determined by analyzing the P-4 loading in the P-21 nanoformulation. P-21 encapsulation efficiency was 97% and P-4 loading was 28%.
Comparison of P-4 Dispersion and Hepatic-targeted Formulations
HPMC-AS is a widely used excipient that has increased solubility. It can form a solid dispersion and inhibit crystallization of the API from the dispersion matrix as well as function as a rate-controlling polymer for a sustained-release dose form. Using P-4/HPMC-AS as a dispersion formulation versus P-21, P-4 showed a greater AUC (1810 ng h/mL), Cmax (154 ng/mL) in blood, and oral bioavailability (F% = 50.7) in mice compared to P-21 AUC (339.5 ng h/mL), Cmax (40 ng/mL) in blood, and oral bioavailability (F% = 22.7). However, greater hepatic delivery of P-4 was obtained with P-21 versus the dispersion formulation of P-4 at both 24 hours and 2 weeks of daily administration (Figure 2 A, B). The effect of P-21 on LDL- C levels in C57BL/6 mice fed high-fat diet showed unexpectedly greater LDL-C efficacy with P-21 versus the dispersion formulation of P-4 after 7 days of daily oral administration (10 mg/kg) (Figure 2C).
Pharmacodynamics Analysis: Test with Nutritionally Induced Hypercholesterolemia Mouse Model
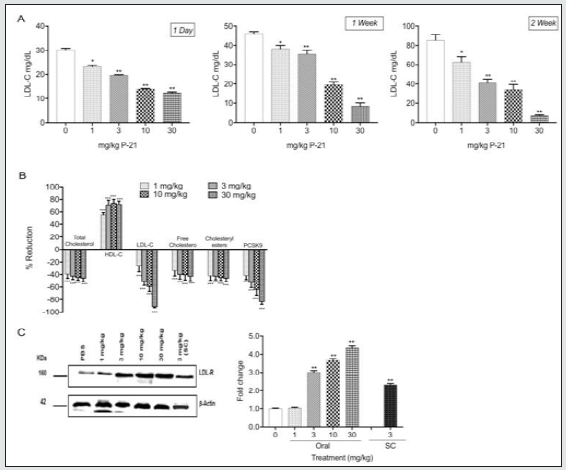
The data demonstrate that P-21 is highly effective at lowering LDL-C. Treatment of mice fed a high-fat diet with P-21 (1, 3, 10, and 30 mg/kg, daily oral dosing) for 2 weeks resulted in a dosedependent decrease in LDL-C ranging from 20-90% (Figure 3A). In addition, P-21 caused a dose-dependent decrease in the levels of plasma PCSK9 and correlated with the decreases in the levels of LDL-C (Figure 3B), [27]. P-21 also regulated the profile of other lipids. It caused a decrease in total serum cholesterol, free cholesterol, and cholesterol ester levels by about 40% and a significant increase in the HDL-C levels of 60-80% (Figure 3B). As expected, P-21 caused dose-dependent increases in the levels of LDLR in the liver (Figure 3C). In view of the foregoing, P-21 clearly demonstrates unexpectedly superior properties for reducing LDL-C.
Figure 2: (A) P-4 levels at 24 hr. Profiles in mice fed high-fat diet after administration of P-21 versus dispersion formulation. Liver tissues were weighed and homogenized in organic solvents (1:1:1 ratio of DMSO: acetonitrile: methanol) along with reserpine as an internal standard. Tissue homogenates were centrifuged at 15,000 x g for 15 min and supernatant was lyophilized and reconstituted in a small but known volume of acetonitrile for injection into the LC/MS/MS. (B) P-4 levels at 24 hr and 2 weeks after daily administration of P-21 in mice fed high-fat diet. The hepatic-targeted delivery of P-21 shows the adjustment of liver levels (2 weeks) after repeated dosing without significant accumulation of drug but with enough residual levels for sustained effects. (C) LDL- cholesterol reduction with the dispersion formulation vs nanocrystal/ hepatic-targeting formulation (P-21) in C57BL/6 mice fed high-fat diet. C57BL/6 mice received 10 mg/kg oral daily for 5 days (dispersion) or 7 days (P-21). Blood plasma was collected and plasma LDL-C levels were measured. The graph shows greater efficacy of P-21 (~60% LDL- lowering) versus the dispersion formulation of P-4 (~20% LDL-lowering). Error bars represent mean ± standard error of the mean.
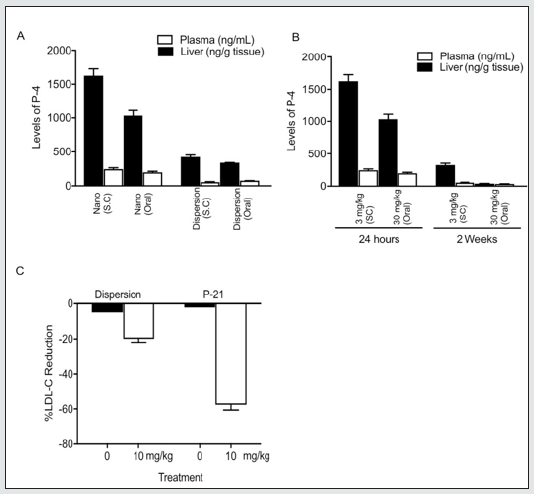
Figure 3: Effect of P-21 on plasma lipid profile in mice fed high-fat diet. Mice (C57BL/6) were divided into groups of 6 animals in each. Control group received PBS; P-21 groups received 1, 3, 10, or 30 mg/kg oral daily for 2 weeks. Blood plasma was collected after 1 day, 1 week, and 2 weeks, and plasma lipids were measured enzymatically. (A) Levels of LDL-C in plasma of high-fat diet mice treated with different doses of P-21 for 1-day,1 week, and 2 weeks. Data show that P-21 exhibited significant dose-dependent LDL-C reduction compared to control. (B) Levels of plasma lipids of high-fat diet mice after 2 weeks. Data show that P-21 exhibited significant reduction of total cholesterol, LDL-C, free cholesterol, cholesterol esters, and PCSK9 with an increase in the HDL-C observed compared to control. (C) Levels of LDL receptor (LDLR) expression from liver extracts versus control. Liver was collected from mice fed high-fat diet treated with PBS and different doses of P-21 (1, 3, 10, and 30 mg/kg oral daily and 3 mg/kg subcutaneous (SC) injection daily) for 2 weeks. Expression of LDLR in mice was analyzed using western blot analysis. Data show an increase in the level of LDLR in animals treated with increased doses of P-21. Error bars represent mean ± standard error of the mean, *P < 0.01, **P < 0.001, ***P <0.0001.
Discussion
According to the AHA, hypercholesterolemia is the only direct atherosclerotic risk factor that is the main cause of mortality worldwide [28-31]. Although blood cholesterol can be lowered using a number of marketed drugs, more than 60% of patients taking these drugs are not achieving the low cholesterol goals [32,33]. It is estimated that 15-20 million patients in the US are not at the desired LDL level and therefore more aggressive treatment of dyslipidemia is needed. Such treatment has come from modulation of the LDLR degradation pathway that has recently been demonstrated with approved mAbs [14,15]. PCSK9 as a therapeutic target is well validated [5,9,14,16]. This is strongly supported by the low plasma LDL-C levels associated with loss-of-function mutations in the PCSK9 gene, indicating that inhibition of PCSK9 by small molecules should make for effective cholesterol-lowering drugs [14,16]. In addition, no safety issues associated with inhibition of PCSK9 have been identified [34]. This is supported by the efficacy and safety obtained from the marketed mAbs, Alirocumab (Praluent) and Evolocumab (Repatha) [14,15]. Recent data from the Odyssey outcome clinical trial for Praluent demonstrates the proof of concept of the association between LDL-C reduction and cardiovascular outcome [35]. This study was conducted on 18,924 patients with acute coronary syndrome for the previous [12] months. Their LDL-C levels were ≥70 mg/dL and they were on maximally tolerated statin therapy. They received subcutaneous injections of Praluent (75 mg) every 2 weeks. After 2.8 years, successful reduction in LDL-C level was achieved (<53.3 mg/dL). This resulted in a further 24% reduction in heart attacks, stenting, and death. Because P-21 works largely through the same mechanism as Praluent, it is anticipated that an oral small molecule like P-21 would be a major participant in the new 2018 AHA/ACC cholesterol guideline paradigm shift.
There is a critical need to discover new, oral drugs for the treatment of high cholesterol that fills the therapeutic gap known for currently marketed drugs. The anti-PCSK9 medicines will be especially useful for those patients who cannot tolerate statins (statin intolerant)[12] or who are statin resistant [11], although we expect our drug to be used alongside statins, in particular for patients who do not achieve cholesterol-lowering goals with statins. Thus far, currently marketed PCSK9 mAbs are facing serious challenges and some major drawbacks that include their high cost [17]. In addition, a new siRNA drug, Inclisiran from Novartis, is expected to soon obtain market approval (NDA submitted to FDA in Dec. 2019) as a twice-annual injectable drug [16], although price, long term safety, and cardiovascular outcomes data, which are known for both Repatha and Praluent, remain to be determined.
The mechanism of a modulator of PCSK9 function could exhibit any one or more of the following characteristics: (a) binds to PCSK9, (b) decreases or blocks PCSK9 interaction with the LDLR, (c) decreases or blocks secretion of PCSK9, (d) decreases or blocks PCSK9-mediated downregulation of the LDLR, (e) inhibits the PCSK9-mediated decrease in LDL blood clearance, (f) increases LDL clearance in media by cultured hepatocytes, (g) increases blood LDL clearance by the liver in vivo, (h) improves patients’ sensitivity to other LDL-lowering drugs, including statins, (i) is synergistic to other LDL- lowering drugs, including statins, and (j) blocks PCSK9 interaction with other yet to be identified factors. In targeting PCSK9, we initially attempted to identify compounds that interfere with the catalytic processing/secretion of PCSK9. We identified orally bioavailable compounds that inhibit PCSK9 processing/secretion without affecting other PCSKs (PCSK4 and PCSK6; unpublished results). The lead compounds showed about 20% LDL-C lowering in mice fed a high-fat, high cholesterol diet. However, because of the existence of many PCSKs (9 are known) and the potential of side effects, we turned our attention to targeting the PCSK9/LDLR interaction where we can identify compounds with high selectivity. In this approach, we utilized virtual screening aimed to develop compounds that interfere with the PCSK9/LDLR interaction. We screened the ZINC all- purchasable database containing over 8 million compounds at the time using the PDB crystal structure (PDB ID: 3BPS) [5] for docking. The pro and V domains and all water molecules were removed (i.e., only the catalytic domain remained). Compounds were docked at the PCSK9 surface where the LDLR (EGF-A domain) binds. Hits confirmation was conducted using our in vitro and cell-based assays. Three classes of compounds were confirmed to upregulate LDLR in a concentration-dependent manner in HEK-293T transfected cells. Our best lead optimization effort focused on benzofuran-2- carboxyanilides series [27], which led to a novel, new lead PCSK9/LDLR antagonist (P-4) [36]. We selected P-4 for in vivo efficacy studies because it had the following properties: it binds to PCSK9 at clinically relevant concentrations with an IC50 = 51 nM, exhibits significant increase in the LDLR upregulation in PCSK9/LDLR recombinant HEK-293 cells, exhibits no effect on the synthesis, processing, and secretion of PCSK9 either in the cells or into the media, and exhibits significant increase in the Dil-LDL uptake at 1.2 μM compound concentration. P-4 was further optimized through extensive formulation efforts that led to our preclinical candidate referred to as P-21.
For in vivo studies, mice have been used extensively to elucidate mechanisms that regulate cholesterol homeostasis because of several advantages such as ease of breeding, large litter size, a short generation time of 9 months, and economies of colony maintenance. The laboratory C57BL/6 mouse is a non-genetically modified model of diet- induced hypercholesterolemia developed through many generations of inbreeding and selection for plasma cholesterol response to high-fat, high cholesterol diet, and high and low responding strains of mice were produced. These strains have normal levels of plasma cholesterol on a basal diet. However, high responding mice exhibit an extremely high LDL cholesterol response when fed a high-fat, high cholesterol diet compared to low responding mice. Although there are important differences between mice and humans in lipoprotein and cholesterol metabolism, nongenetically modified mice have high levels of HDL cholesterol and low levels of LDL cholesterol, whereas humans have low levels of HDL cholesterol and high levels of LDL cholesterol. The difference in lipid profiles between mice and humans is due to absence of the cholesteryl ester transfer protein (CETP) in mice [37]. In normal mice lacking CETP, more than 80% of plasma cholesterol is carried on HDL, so mice with high levels of HDL cholesterol are resistant to hypercholesterolemia and atherosclerosis. Yet, mouse PCSK9 shares the highest rodent homology to human with more than 80% identity, which confirms that the C57BL/6 is our best rodent model.
Many different formulations (conventional and nano formulations) were tested to ensure our lead antagonists are as potent as the mAb. To improve their efficacy, we used a nanotechnology platform for P-4 and its analogs for differential targeted delivery to the liver to maximize efficacy and minimize its systemic distribution. We used nanoparticles comprising P-4 and hepatic targeting moieties such as Alginic acid for liver-targeted delivery. Nano-hepatic targeted P-4 can be used as a monotherapy, especially for the statin-intolerant patient population, or combined with a statin or other lipid lowering substance for patients who are resistant to lipid lowering. The combined drugs (e.g., PCSK9 antagonist and lipid lowering substance) could also be nano-encapsulated into hydrophobic nanoparticles. Examples of hydrophobic nanoparticles include amine- modified poly- (lactic-co-glycolic acid) (PLGA), docosahexaenoic acid (DHA), eicosatetraenoic acid (EPA), or EPA/DHA combinations, or solid lipid nanoparticles (SLNs). Encapsulation of P-4 into hydrophobic PLGA with or without chitosan and polyethylene glycol (PEG), chitosan-EPA, chitosan-DHA or chitosan-EPA/DHA nanoparticles, as well as SLNs conjugated to hepatic targeting moieties such as Glycyrrhetinic acid, Lactobionic acid, and Alginic acid, are also exemplified [38].
To our knowledge, no small molecular antagonist against PCSK9 has yet been taken into clinic, but we are aware of at least two other companies in early-stage development. There are many potential advantages of Shifa’s oral P-21 over injectable mABs (Praluent and Repatha) therapies. Advantages include that doctors, patients, and payers prefer oral to injectable drugs, it will have higher profit margins, no contamination issues are anticipated, high stability is observed at room temperature, and no refrigeration is needed. P-21 could be formulated as an oral fixed-dose combination and has no anticipated immunogenicity or injection site complications. Therefore, Shifa’s P-21 oral small molecule will have a significant competitive advantage. Thus, identifying an orally bioavailable, small molecule that interferes with the PCSK9 mechanism of action could be revolutionary.
Conclusion
P-21 showed outstanding potency against PCSK9 in mice fed a high-fat diet. Our data show that P-21 is highly effective at lowering LDL-C. Oral administration of P-21 alone in mice fed a high-fat diet resulted in close to 90% LDL-C lowering. The LDL-C lowering effect of P-21 is as potent as the mAbs, Praluent, or Repatha. Significant reduction in plasma and liver PCSK9 was achieved. P-21 is safe because no liability issues were observed in vitro in multiple cellbased assays and in an in vivo animal model.
Acknowledgement
We appreciate the excellent manuscript editing by Dr. Kelly A. Keating, science editor/medical writer at the Pharmaceutical Research Institute, ACPHS, Rensselaer, NY.
Sources of Funding
This research was supported by SBIR grant # R44HL137449 and R44HL150923.
References
- Abd Alamir M, Goyfman M, Chaus A, Dabbous F, Tamura L, Sandfort, et al. (2018) The correlation of dyslipidemia with the extent of coronary artery disease in the multiethnic study of atherosclerosis. J Lipids 2018: 5607349.
- Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S (2016) Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res 118(4): 535-546.
- Abifadel M, Varret M, Rabes JP, Delphine Allard, Khadija Ouguerram, et al. (2003) Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 34(2): 154-156.
- Benjannet S, Rhainds D, Essalmani R, Janice Mayne, Louise Wickham, et al. (2004) NARC-1/PCSK9 and its natural mutants: Zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem 279(47): 48865-48875.
- Kwon HJ, Lagace TA, McNutt MC, Horton JD, Deisenhofer J (2008) Molecular basis for LDL receptor recognition by PCSK9. Proc Natl Acad Sci U S A. 105(6): 1820-1825.
- Zhao Z, Tuakli-Wosornu Y, Lagace TA, Kinch L, Grishin NV, et al. (2006) Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet 79(3): 514- 523.
- Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, et al. (2005) Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet 37(2): 161-165.
- Wang Y, Huang Y, Hobbs HH, Cohen JC (2012) Molecular characterization of proprotein convertase subtilisin/kexin type 9-mediated degradation of the LDLR. J Lipid Res 53(9): 1932-1943.
- Horton JD, Cohen JC, Hobbs HH (2009) PCSK9: A convertase that coordinates LDL catabolism. J Lipid Res. 50 Suppl: S172-S177.
- Lin Y, Mousa SS, Elshourbagy N, Mousa SA (2010) Current status and future directions in lipid management: Emphasizing low-density lipoproteins, high-density lipoproteins, and triglycerides as targets for therapy. Vasc Health Risk Manag 6: 73-85.
- Karalis DG, Victor B, Ahedor L, Liu L (2012) Use of lipid-lowering medications and the likelihood of achieving optimal LDL-cholesterol goals in coronary artery disease patients. Cholesterol 2012: 861924.
- Toth PP, Patti AM, Giglio RV, Nikolic D, Castellino G, et al. (2018) Management of statin intolerance in 2018: Still more questions than answers. Am J Cardiovasc Drugs 18(3): 157-173.
- Ungar L, Sanders D, Becerra B, Barseghian A (2018) Percutaneous coronary intervention in familial hypercholesterolemia is understudied. Front Cardiovasc Med 5: 116.
- (2016) Amgen. FDA approves first and only single monthly injection for a PCSK9 inhibitor.
- (2015) Sanofi. Sanofi and Regeneron announce FDA approval of Praluent® (alirocumab) injection, the first PCSK9 inhibitor in the U.S., for the treatment of high LDL cholesterol in adult patients.
- (2020) Taylor NP. Novartis posts pivotal data ahead of FDA decision on $9.7B bet.
- (2020) Campbell P. Bempedoic acid and ezetimibe combination approved by FDA for lowering LDL-c.
- (2018) Pagliarulo N. Amgen cuts US Repatha price 60% amid market pressure.
- Imai K, Takaoka A (2006) Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer 6(9): 714-727.
- Ullah N, Khan S, Ahmed S, Govender T, Faidah HS, et al. (2018) Dexibuprofen nanocrystals with improved therapeutic performance: Fabrication, characterization, in silico modeling, and in vivo evaluation. Int J Nanomedicine 13: 1677-1692.
- Cheng Y, Yu S, Wang J, Qian H, Wu W, et al. (2012) In vitro and in vivo antitumor activity of doxorubicin-loaded alginic-acid-based nanoparticles. Macromol Biosci 12(10): 1326-1335.
- Zuccari G, Baldassari S, Ailuno G, Turrini F, Alfei S, et al. (2020) Formulation strategies to improve oral bioavailability of ellagic acid. Appl Sci 10(10): 3353.
- Sudha T, Bharali DJ, Yalcin M, Darwish NH, Debreli Coskun M, et al. (2017) Targeted delivery of paclitaxel and doxorubicin to cancer xenografts via the nanoparticle of nano-diamino-tetrac. Int J Nanomedicine 12: 1305-1315.
- Patel VR, Agrawal YK (2011) Nanosuspension: An approach to enhance solubility of drugs. J Adv Pharm Technol Res 2(2): 81-87.
- Vinarov Z, Katev V, Radeva D, Tcholakova S, Denkov ND (2018) Micellar solubilization of poorly water-soluble drugs: Effect of surfactant and solubilizate molecular structure. Drug Dev Ind Pharm 44(4): 677-686.
- Masina N, Choonara YE, Kumar P, du Toit LC, Govender M, et al. (2017) A review of the chemical modification techniques of starch. Carbohydr Polym 157: 1226-1236.
- Elshourbagy N, Godugu K, Fujioka K, Li W, Meyers H, et al. (2019) Abstract 9947: Oral protease proprotein convertase subtilisin-like kexin type 9 antagonist: Direction to the clinic as a treatment for hypercholesterolemia. Circulation. 140
- Emelia JB, Paul M, Alvaro A, Marcio S Bittencourt, Clifton W Callaway, et al. (2019) Heart disease and stroke statistics—2019 update: A report from the American Heart Association. Circulation. 139(10): e56- e528.
- (2020) Centers for Disease Contol and Prevention. High cholesterol facts.
- (2020) Centers for Disease Control and Prevention. Heart disease facts.
- (2017) American Heart Association. Cardiovascular disease costs will exceed $1 trillion by 2035, warns the American Heart Association.
- (2001) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 285: 2486-2497.
- Robertson S (2019) Statins provide no benefit for 50 percent of patients, say researchers.
- Maxwell KN, Breslow JL (2004) Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci U S A. 101(18): 7100-7105.
- American College of Cardiology (2018) ODYSSEY outcomes: Results suggest use of PCSK9 inhibitor reduces CV events, LDL-c in ACS patients .
- Elshourbagy N, Godugu K, Fujioka K, Li W, Meyers H, et al. (2019) Late-Breaking Basic Science Abstracts From the American Heart Association’s Scientific Sessions 2019: Abstract 20993 efficacy and safety data on novel oral proprotein convertase subtilisin-kexin type 9 antagonist. Circ Res. 125(12): e99-e111.
- Madariaga YG, Cardenas MB, Irsula MT, Alfonso OC, Caceres BA, et al. (2015) Assessment of four experimental models of hyperlipidemia. Lab Anim (NY) 44(4): 135-140.
- Mousa S, Elshourbagy N, Meyers H, Abdel-Meguid S (2020) Anti-proprotein convertase subtilisin kexin type 9 (anti-pcsk9) nano-formulation of compounds and methods of using the same. Patent WO2020097016A1.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...


