
Lupine Publishers Group
Lupine Publishers
Menu
Case Report(ISSN: 2770-5447) 
Concomitant LVAD Implantation and Thoracic Surgery. Clinical Decision Making and Surgical Challenges Volume 2 - Issue 4
Massimo Maiani1, Sandro Sponga1, Anita Brondani2, Vincenzo Tursi1, Angelo Morelli1, Andrea Lechiancole*1, Daniela Piani1, Mauro Driussi1, Helena Ortis1 and Ugolino Livi1
- 1Cardiothoracic Department, University Hospital of Udine, Italy
- 2Department of Anesthesia, University Hospital of Udine, Italy
Received: November 21, 2019; Published: December 10, 2019
Corresponding author: Andrea Lechiancole, Cardiothoracic Department, University Hospital of Udine, P le SM della Misericordia 1533100 Udine, Italy
DOI: 10.32474/ACR.2019.02.000144
Abstract
In the last years, left ventricular assist device (LVAD) indications have significantly broadened including candidates with multiple comorbidities even requiring combined surgical strategy. We present a case of a 56-year old patient affected by postischemic dilated cardiomyopathy in whom a lung nodule diagnosed during preoperative CT-scan could have contraindicated LVAD destination therapy. The patient underwent through a median sternotomy concomitant LVAD implantation on cardiopulmonary bypass followed by an atypical resection of the anterior part of the right lower lobe lung. A multidisciplinary, step-by-step approach to reduce the risk of right ventricular failure, bleeding and infections is presented.
Keywords: LVAD implantation; non-cardiac surgery; atypical lung resection
Introduction
Indications to continuous flow left ventricular assist devices (CF-LVAD) have significantly broadened over the last decade considering even old patients with cardiac and extra-cardiac comorbidities [1]. Concomitant cardiac procedures to LVAD implantation are well described since nowadays up to 35% of implantations require concomitant cardiac surgery including valvular surgery, coronary artery bypass grafting, ventricular arrhythmias ablation, and atrial septal defects repair [1]. But the impact of these concomitant procedures is not well studied and guidelines are lacking. In particular for non-cardiac surgery experiences are limited to case series and the decision-making is driven on a single patient basis. To our knowledge, concomitant LVAD implantation and lung surgery has not been described before. We report the case of a patient who underwent a second generation CF-LVAD implantation followed by an atypical right lower lobe resection.
Case Report
A 56-year-old man mildly obese and previous heavy smoker, presented with post-ischemic dilated cardiomyopathy and severely decompensated heart failure (orthopnea, dyspnea, fluid retention, and weight gain) with left ventricular ejection fraction (EF) of 17%. He also showed non-reversible post-capillary pulmonary hypertension and right ventricular (RV) dysfunction: Tricuspid Annular Plane Systolic Excursion (TAPSE) of 10 mm, RV Fractional Area Change (RVFAC) of 20%, Right Ventricular Stroke Work Indexed (RVSWI) of 400 mmHg/ml/m2 and a central venous pressure to wedge pressure ratio of 0.8. Few days after admission the patient developed hemoptysis. A 15-mm enhanced contrast lung nodule in the latero-basal segment of the right lower lobe (RLL) with a maximum standardized uptake value of 8, was demonstrated at the Positive Emission Tomography Scan (Figure 1A). The CT-scan showed a nodule suspicious for neuroendocrine tumor because of early contrast enhancement and a regular profile. Due to its peripheral position the nodule could not be reached with a transbronchial biopsy and a percutaneous computer tomography guided biopsy was considered to be too high risk. The pulmonary function tests were normal despite the presence of centrilobular and paraseptal emphysema.
Figure 1: (A)Computer Tomography and Positron Emission Tomography scan showing RLL enhanced contrast nodule; (B) manual palpation of lung nodule with deflated lung; (C) wedge resection of the RLL; (D) histology of the lung lesion showing a complex artero-venous malformation.
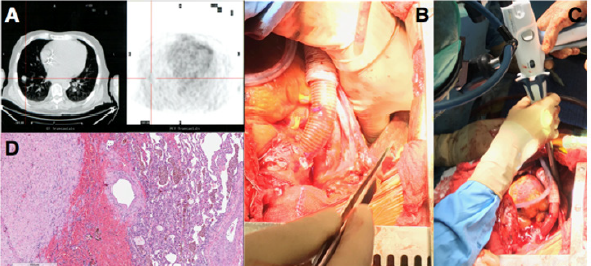
He showed a positive clinical response to an aggressive diuretic therapy with significant reduction in weight and fluid retention, and improvement in right ventricular function (TAPSE 17 mm, RVFAC 33%, RVSWI 600 mmHg/mL/m2, central venous pressure to wedge pressure ratio of 0.4) which allowed to list the patient for an LVAD implantation. Considering right ventricular improvement, favorable position of the lung nodule and the complex coagulation management of a staged approach a combined procedure was preferred. A 24 hours infusion of levosimendan was completed in the immediate preoperative period. In consideration of the presence of the pulmonary lesion, we decided for a destination therapy configuration of the Jarvik 2000 (Jarvik Heart, Inc, New York, NY, USA) with an intraoperative retroauricolar implantation of the pedestal. After fixation of the pedestal, the pericardial space was approached via a median longitudinal sternotomy. Once the driveline was tunneled, LVAD implantation inside the left ventricle apex was performed on cardiopulmonary bypass (CPB) and beating heart, the outflow conduit was sutured to the ascending aorta. The progression from CPB to LVAD level 2 support was facilitated by continuous infusion of epinephrine (0.04 mcg/kg/min), norepinephrine (0.05 mcg/kg/min) and 20 ppm of inhaled nitric oxide to facilitate RV function.
When surgical and medical hemostasis were carefully achieved, one lung ventilation with 5 mL/Kg tidal volumes was initiated and a positive end expiratory pressure of 8 cmH2O, inhaled nitric oxide was increased to 30 ppm. Even if the PaO2/FiO2 ratio was 100 mmHg and the PaCO2 was 47 mmHg, the TEE showed normal RV function with a pulmonary artery systolic pressure of 40 mmHg. RLL exposure was achieved through the mediastinal pleura via the median sternotomic access. Manual palpation with inflated and noninflated lung allowed nodule identification and wedge resection of the RLL with an Echelon Flex 60 Endopatch (Ethicon Endo Surgery Inc., Cincinnati, OH) (Figure 1 B,C). Pathological examination demonstrated a complex artero-venous malformation (Figure 1D). The patient had an uneventful postoperative course with weaning from mechanical ventilation and inotropic support within the first 36 postoperative hours. He was discharged from the postoperative intensive care unit on day 6 and on day 17 to a rehabilitation center. Since the lung lesion was benign the patient was eligible to enter the heart transplantation list discharged and strictly monitored with a telemedicine program previously described [2].
Discussion
The population of patients eligible for LVAD implantation has broadened over the last years and thanks to the reported improvements in mid-term outcomes and quality of life, the number of patients scheduled for a concomitant non-cardiac surgery intervention has progressively increased [3]. Planning a combined procedure in these fragile patients requires a thorough multidisciplinary approach, a strict preoperative medical optimization and the design of an effective and safe surgical procedure. Few cases of thoracic surgery in patients with CFLVAD have been published [4,5]. In this case, surgical plan was carefully defined in consideration of the major perioperative risks. Great attention was paid to RV function and preservation since hemodynamic changes following LVAD implantation are difficult to predict and have a huge impact on results [6,7]. Since no data have been published on the relationship between the amount of lung parenchyma excised and the variation of pulmonary resistances and thus of right ventricular afterload, we have chosen a step-bystep approach. An atypical lung resection was first performed. A RLL lobectomy would be a second surgical step only in case of demonstrated malignancy, minimizing in this way right ventricular impact and allowing a gradual adaptation of the RV to increased pulmonary resistances. During the procedure RV function was evaluated through surgical visual inspection, TEE and right heart catheterization allowing for immediate pump speed variations or pharmacological support titration.
The delicate interaction between RV, mechanical ventilation and hypoxia has been largely described in the population of patients suffering from acute respiratory distress syndrome and also during the intraoperative management of one lung ventilation for thoracic surgery, but it has never been studied in the context of LVAD support [8]. Indeed, RV disfunction after LVAD implantation is a life threating complication that can occur in 20 to 50% of patients [9] especially in the presence of high pulmonary vascular resistances. The RV showed a positive response to the increased venous return, to the geometrical adaptation of the interventricular septum and the augmented pulmonary vascular resistances. The favorable anatomical position of the lung nodule allowed to employ the median sternotomy for both LVAD implantation and lung resection. Concomitant procedure reduced the perioperative bleeding and infective risks. This case underlines the crucial role of a multidisciplinary approach, a preoperative medical optimization, a step-by-step surgical plan and a multimodal right ventricular evaluation.
Discussion
- Maltais S, Haglund NA, Davis ME, Aaronson KD, Pagani FD, et al. (2016) Outcomes after concomitant procedures with left ventricular assist device implantation: implications by device type and indication. ASAIO Journal 62(4): 403-409.
- Sponga S, Bagur R, Livi U (2018) Teleconsultation for left ventricular assist device patients: a new standard of care. Eur J Heart Fail 20(4): 818-821.
- Lee1 S, Young JB, Naftel DC, Kirklin JK, Moazami N, et al. (2015) Impact of concomitant cardiovascular surgeries at the time of CF-LVAD implantation: an INTERMACS analysis. J Heart Lung Transplantation 34 (4S): S153-154.
- Murakawa T, Murayama T, Nakajima J, Ono M (2011) Lung lobectomy in a patient with an implantable left ventricular assist device. Interactive Cardiovascular Thoracic Surgery 13(6): 676-678.
- Wei B, Takayama H, Bacchetta MD (2009) Pulmonary lobectomy in a patient with a left ventricular assist device. Ann Thorac Surg 87(6): 934-1936.
- Bellavia D, Iacovoni A, Scardulla C, Moja L, Pilato M, et al. (2017) Prediction of right ventricular failure after ventricular assist device implant: systematic review and meta-analysis of observational studies. Eur J Heart Failure 19(7): 926-946.
- Sponga S, Ivanitskaia E, Potapov E, Krabatsch T, Hetzer R, et al. (2012)Preoperative treatment with levosimendan in candidates for mechanical circulatory support. ASAIO J 58(1): 6-11.
- Zochios V, Parhar K, Tunnicliffe W, Roscoe A, Gao F (2017) The right ventricle in ARDS. Chest 152(1): 181-193.
- Lo C, Murphy D, Summerhayes R, Quayle M, Burrell A, et al. (2015) Right ventricular failure after implantation of continuous flow left ventricular assist device: analysis of predictors and outcomes. Clin Transplant 29(9): 763-770.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...


