
Lupine Publishers Group
Lupine Publishers
Menu
Research Article(ISSN: 2770-5447) 
Successful Percutaneous Coronary Intervention of the Right Coronary Artery After a Self-Expandable Transcatheter Aortic Valve Replacement: A Case Report Volume 3 - Issue 5
Rohan Madhu Prasad*1, Zulfiqar Qutrio Baloch2, Adolfo Martinez Salazar3, Abdullah Al-abcha4, Sajjad Ali5, FNU Samreen6, Abbas Ali7 and George S Abela8
- 1-4,6Department of Internal Medicine, Michigan State University - Sparrow Hospital, MI, USA
- 5Department of Internal Medicine, Northern Light Health, USA
- 7,8Department of Cardiology, West Virginia University, USA
Received:January 14 ,2022; Published:January 25, 2022
Corresponding author:Rohan Madhu Prasad, Sparrow Clinical Research Institute, 1200 E Michigan Ave, Suite 550, Lansing, MI 48912, USA.
DOI: 10.32474/ACR.2019.03.000174
Abstract
Background: Patients with previous transcatheter aortic valve replacement (TAVR) can present with an acute coronary syndrome that requires coronary angiography and percutaneous coronary intervention (PCI). However, these procedures can be difficult if the patient has a self-expandable TAVR as it covers the coronary arteries.
Case Report:An 87-year-old female with aortic valve stenosis and self-expanding TAVR presented with syncope, shortness of breath, and chest pain. Electrocardiography showed sinus bradycardia, left axis deviation, and T-wave inversion in the lateral leads. Troponin levels trended up to 37.8 pg/mL, which was consistent with a non-ST elevation myocardial infarction. A pre-procedural coronary angiography at that time also revealed no significant stenosis. Shared decision-making was performed and the patient underwent a diagnostic right and left coronary angiography with possible PCI. A pre-procedural computed tomography chest visualized the prosthetic valve location. The coronary angiography was performed via the Judkins technique, which showed a totally occluded proximal right coronary artery. Thereafter, a JR4 guide catheter was used to engaged the obstruction, a CHOICE floppy wire was advanced across the occlusion, a balloon was deployed to achieve patency, and a drug-eluting stent was placed. The patient was discharged two days after the procedure on dual antiplatelet therapy.
Conclusion: Since the self-expanding TAVRs are located supra-annularly, the diamond cells may block the entrance of the coronary arteries to the PCI. With an anatomical picture through a computed angiography of the chest and procedural experience, successful coronary angiography and selective PCI is possible in patients with a TAVR
Keywords:Percutaneous coronary intervention; transcatheter aortic valve replacement; right coronary artery; coronary arterial disease; case report
Introduction
The guideline-based treatment for symptomatically critical aortic stenosis is either surgical aortic valve replacement (SAVR) or transcatheter aortic valve replacement (TAVR) via percutaneously [1]. A meta-analysis of 33 studies revealed that the prevalence of patients with calcified aortic stenosis and coronary arterial disease (CAD) was 37% [2]. Moreover, TAVR patients with CAD have higher 30-day and overall mortality rates, as compared to those without CAD. Therefore, coronary angiography with possible percutaneous coronary intervention (PCI) is a requirement for TAVR candidates [3]. With a successful PCI, there is decreased myocardial ischemia and increased coronary blood flow, which enhances the recovery from the TAVR [4]. However, patients with previous TAVR can present with an acute coronary syndrome that requires a coronary angiography with possible PCI. Large and medium-sized case series have reported the prevalence of CAD after TAVR as 0.6-4.1% [5,6]. These procedures can prove to be difficult and challenging, especially if the patient has a self-expandable TAVR as it covers the coronary arteries. Additionally, PCI of the right coronary artery is only occasionally successful at 50%, 16/32 [7-9].
Case Presentation
An 87-year-old female presented for a syncopal episode, shortness of breath, and chest pain. She had a medical history of aortic valve stenosis status post TAVR, hypertension, chronic kidney disease, chronic anemia, and osteoarthritis. On admission, the patient was alert, oriented, and vitally stable. The patient stated that she briefly passed out while sitting, but denies falling or any injuries. She admits that the shortness of breath and chest pain occur frequently, especially at exertion. Chest auscultation revealed left-sided decreased breath sounds. Electrocardiography showed sinus bradycardia, left axis deviation, and T-wave inversion in the lateral leads. Troponin levels were initially 0.07 pg/mL, but trended up to 37.8 pg/mL (reference range <19 pg/mL). The patient was diagnosed with having a non-ST elevation myocardial infarction (NSTEMI). In regards to the TAVR, a chart review showed that three years ago a Core Valve (Medtronic, Minneapolis, Minnesota, USA) was placed. A pre-procedural coronary angiography at that time also revealed no significant stenosis. Echocardiography was done and revealed inferior wall hypokinesis due to aortic valve stenosis, but the valve had normal function.
Shared decision-making was performed with the patient and eventually a diagnostic right and left coronary angiography with possible interventions was agreed upon to evaluate her vessels. A pre-procedural computed tomography scan of the chest was obtained to visualize the location of the valve prosthetic. The right femoral artery was directly entered, and a 6-French sheath was placed. The coronary angiography was performed via a 6-French Judkins technique, which showed a totally occluded proximal right coronary artery (Figure 1). Therefore, a JR4 guide catheter was advanced and engaged the obstruction. After administering intravenous heparin, a CHOICE floppy wire was advanced across the total occlusion and a 2.5 mm x 8 mm balloon was serially deployed to achieve patency (Figure 2). Angiography was then performed with a 2.75 mm x 24 mm drug-eluting stent to achieve a peak pressure of 14 atmospheres (Figure 3). Femoral angiography was then performed, and an Angio-Seal device was placed. A cine fluoroscopy confirmed the correct positioning of the metallic stent. Post-intervention angiography showed a distal TIMI grade 3 flow, but no residual stenosis or evidence of distal embolization was noted. The patient was started on dual antiplatelet therapy of aspirin and clopidogrel. The patient’s initial symptoms resolved within two days after the procedure and were discharged home on day three of hospitalization. She was instructed to continue taking dual antiplatelet therapy along with her other medications. Followup visits were scheduled with her primary care physician at two weeks and cardiologist at one month, which were unremarkable.
Figure 1: Coronary angiography showing total occlusion of the right coronary artery
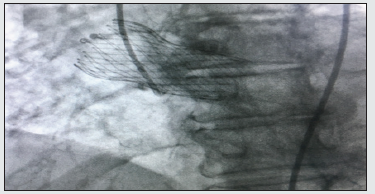
Figure 2:Coronary angiography showing successful placement of the guidewire

Figure 3:Coronary angiography showing successful placement of the stent.

Discussion
Patients with previous TAVR are at risk of developing CAD and acute coronary syndrome. Post-TAVR coronary ostial stenosis occurs most often in the left main coronary artery, but can be seen in the right coronary artery [10-12]. Numerous pathophysiological mechanisms have been suggested for the increased risk of CAD in TAVR patients. Firstly, there is an inherent risk of micro-injuries and local hyperplastic reaction from the cardioplegic fluid infusion pressure and vessel overdilation by the catheter tip during TAVR [13]. Autopsy of post-TAVR subjects reported fibrous thickening in the aortic root proximal to the coronary artery [14]. Although histological examinations have not confirmed the presence of atherosclerosis in coronary ostial stenosis [12], the stenosis may have an immunological reaction to the heterograft [13]. Turbulent flow near the prosthetic valves can also aggravate intimal thickening and result in fibrous proliferation near the aortic root and ultimately coronary ostial stenosis [14]. Finally, conventional cardiovascular risk factors have been related to aortic stenosis and CAD [15].
The balloon-expanding Sapien valves (Edwards Lifesciences, Irvine, California, USA) sit in the subcoronary position or at the level of the coronary ostia. Therefore, they do not obstruct the coronary arteries and successful PCI post-TAVR has been demonstrated [7,16]. On the other hand, the self-expanding Core Valve series (Medtronic, Minneapolis, Minnesota, USA) is located supra-annular and the diamond cells may block the entrance of the coronary arteries [7,16]. The catheter can also be entrapped by the prosthesis struts [16]. However, due to their central concavity of the prosthesis struts, selective coronary catheterization may be possible [7,16,18]. Selective coronary angiography of the RCA in patients with a Core Valve had a success rate of 50% (16/32) [7]. Another scenario is patients with valve-in-valve procedures, as they have a high rate of coronary obstruction requiring PCI [19]. The type of valve that is to be used on a patient with severe aortic stenosis relies on many factors. However, the potential need for coronary procedures should not be considered for this purpose [20].
Acute coronary syndrome was present in 1 out of 10 patients after a median follow-up of 2 years post-TAVR. The most common type of presentation was found to be an NSTEMI, either type I or II. Additionally, most coronary events were related to new lesions that were not present or insignificant at the time of coronary angiography prior to the TAVR [21,22]. ACS was associated with poor in-hospital and late prognosis. However, PCI at the ACS time was associated with a 50% reduction in the risk of all-cause death [21]. Selecting a proper access site for cardiac catheterization procedures is another important part of these cases. It is not uncommon to see very tortuous iliac and femoral arteries in patients with severe aortic valve stenosis. In these patients, radial or brachial artery use should always be considered [23]. With the indication for TAVR evolving to a younger generation with longer life expectancies, one would expect to do more coronary angiographies after TAVR in the future [24]. This is one of the reasons why it is crucial to completely understand the 3-dimensional geometric interaction of all the components (the valve prosthesis, the aortic root, and coronary ostia). This can help to be prepared and facilitate coronary angiography and PCI if needed on any patient with prior TAVR placement [25]. A CT analysis can be useful to confirm anatomical factors associated with difficult coronary engagement such as the position of coronary ostium in relation to valve skirt, sinotubular junction dimensions, sinus of Valsalva height, leaflet calcification, and commissural post orientation [26]. This case report illustrates that patients can develop significant coronary ostial stenosis after having a TAVR. Additionally, successful, and safe coronary angiography and PCI of a totally occluded RCA is possible, especially with enough procedural experience and when guided by computed tomography of the chest.
Conclusion
Patients with severe aortic stenosis who are managed with TAVR continue to have a risk of developing coronary artery disease and acute coronary syndrome. Challenging selective coronary catheterization can happen in patients with low coronary ostia and TAVRs with high implants and self-expandable valves. Ideally, these patients should receive a computed tomography scan of the chest to evaluate if their anatomy makes them candidates for PCI. This can guide on what kind of approach would be appropriate for the patient and will eventually improve the outcomes. However, further randomized controlled trials should be conducted where patients with self-expandable TAVR undergo diagnostic coronary angiography.
References
- Siqueira D, Abizaid A, Arrais M, Sousa JE (2012) Transcatheter aortic valve replacement in elderly patients. J Geriatr Cardiol 9(2): 78-82.
- Mauter GC, Roberts WC (1992) Reported frequency of coronary arterial narrowing by angiogram in patient with valvular aortic stenosis. Am J Cardiol 70(4): 539-540.
- Dewey TM, Brown DL, Herbert MA, Culica D Lars G Svensson, et al. (2010) Effect of concomitant coronary artery disease on procedural and late outcomes of transcatheter aortic valve implantation. Ann Thorac Surg 89(3): 758-767.
- Dvir D, Assali A, Spargias K, Kornowski R (2009) Percutaneous aortic valve implantation in patients with coronary artery disease: review of therapeutic strategies. J Invasive Cardiol 21(12): 237-241.
- Thomas M, Schymik G, Walther T, Himbert D, Lefevre T, et al. (2010) Thirty-day results of the SAPIEN aortic bioprosthesis European outcome (SOURCE) registry: a European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 122(1): 62-69.
- Bartorelli AL, Andreini D, Sisillo E, Tamborini G, Fusari M, et al. (2010) Left main coronary artery occlusion after percutaneous aortic valve implantation. Ann Thorac Surg 89(3): 953-935.
- Tanaka A, Jabbour RJ, Testa L, Claudia Fiorina , Marianna Adamo, et al. (2019) Incidence, Technical Safety, and Feasibility of Coronary Angiography and Intervention Following Self-expanding Transcatheter Aortic Valve Replacement. Cardiovasc Revasc Med 20: 371-375.
- Geist V, Sherif MA, Khattab AA (2009) Successful percutaneous coronary intervention after implantation of a CoreValve percutaneous aortic valve. Catheter Cardiovasc Interv 73(1): 61-67.
- López-Otero D, Trillo-Nouche R, Souto-Castro P, Gonzalez-Juanatey JR (2010) Percutaneous coronary intervention in a patient with a CoreValve prosthesis. Rev Esp Cardiol 63(9): 1108-1109.
- Sethi GK, Scott SM, Takaro T (1979) Iatrogenic coronary artery stenosis following aortic valve replacement. J Thorac Cardiovasc Surg 77: 760-767.
- Pande AK, Gosselin G (1995) Iatrogenic left main coronary artery stenosis. J Invasive Cardiol 7(6): 183-187.
- Ziakas AG, Economou FI, Charokopos NA, Antonios A Pitsis, Despina G Parharidou, et al. (2010) Coronary ostial stenosis after aortic valve replacement. Treatment of 2 patients with drug-eluting stents. Tex Heart Inst J 37(4): 465-468.
- Tukiji M, Akasaka T, Wada N, Noriko Okahashi, Teruyoshi Kume, et al. (2004) Bilateral coronary ostial stenosis after aortic valve replacement with freestyle stentless bioprosthesis: a case report. J Cardiol 44(5): 207-213.
- Roberts WC, Morrow AG (1967) Late postoperative pathological findings after cardiac valve replacement. Circulation 35 (4 Suppl): I48-162.
- Ortlepp JR, Schmitz F, Bozoglu T, Hanrath P, Hoffmann R (2003) Cardiovascular risk factors in patients with aortic stenosis predict prevalence of coronary artery disease but not of aortic stenosis: An angiographic pair matched case-control study. Heart 89(9): 1019-1022.
- Ferreira-Neto AN, Puri R, Asmarats L, Eric Dumont, Josep Rodés-Cabau, et al. (2019) Clinical and Technical Characteristics of Coronary Angiography and Percutaneous Coronary Interventions Performed before and after Transcatheter Aortic Valve Replacement with a Balloon-Expandable Valve. J Interv Cardiol 3579671: 1-9.
- Harhash A, Ansari J, Mandel L (2016) STEMI After TAVR: Procedural Challenge and Catastrophic Outcome. JACC Cardiovasc Interv 9(13): 1412-1913.
- Jabbour RJ, Colombo A, Latib A (2020) Looking Toward the Post-TAVR Period and Keeping Options Open for Easy Coronary Access. JACC Cardiol Interv 13(8): 951-953.
- Dvir D, Webb J, Brecker S, Antonio Colombo, Fleur Descoutures, et al. (2012) Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the Global Valve-in-Valve Registry. Circulation 126(19): 2335-2344.
- Boukantar M, Gallet R, Mouillet G, Abdelkaoui Belarbi , Vladimir Rubimbura, et al. (2017) Coronary Procedures After TAVI With the Self-Expanding Aortic Bioprosthesis Medtronic CoreValve™, Not an Easy Matter. J Interv Cardiol 30(1): 56-62.
- Faroux L, Munoz-Garcia E, Serra V, Quentin Fischer, Gabriela Veiga, et al. (2020) Acute Coronary Syndrome Following Transcatheter Aortic Valve Replacement. Circ Cardiovasc Interv 13(2): e008620.
- Vilalta V, Asmarats L, Ferreira-Neto AN, Leonardo de Freitas Campos Guimarães, Thomas Couture, et al. (2018) Incidence, Clinical Characteristics, and Impact of Acute Coronary Syndrome Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 11(24): 2523-2533.
- Aikawa Y, Kataoka Y, Kanaya T, Makoto Amaki, Yoshio Tahara, et al. (2018) Procedural challenge of coronary catheterization for ST-segment elevation myocardial infarction in patient who underwent transcatheter aortic valve replacement using the CoreValveTM. Cardiovasc Diagn Ther 8(2): 190-195.
- Htun WW, Grines C, Schreiber T (2018) Feasibility of coronary angiography and percutaneous coronary intervention after transcatheter aortic valve replacement using a Medtronic™ self-expandable bioprosthetic valve. Catheter Cardiovasc Interv 91(7): 1339-1344.
- Yudi MB, Sharma SK, Tang GHL, Kini A (2018) Coronary Angiography and Percutaneous Coronary Intervention After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 71(12): 1360-1378.
- Khan M, Senguttuvan NB, Krishnamoorthy P, Yuliya Vengrenyuk , Gilbert H L Tang, et al. (2020) Coronary angiography and percutaneous coronary intervention after transcatheter aortic valve replacement with Medtronic self-expanding prosthesis: Insights from correlations with computer tomography. Int J Cardiol 317: 18-24.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...


