Lupine Publishers Group
Lupine Publishers
Menu
Review Article(ISSN: 2641-6875) 
Nutritional Genomics - An Overview of CRISPR/Cas9 Genome Editing System on Nutraceuticals Volume 2 - Issue 4
Milap Mistry1,2, Aayushi Patel1,2, Shrina Patel1,2, Laura Branch1, Shobha Potlakayala1, and Sairam Rudrabhatla1*
- 1Penn State Harrisburg, Middletown, PA, United States of America
- 2Equal Contribution
Received:December 14, 2021; Published:January 6, 2022
*Corresponding author: Sairam Rudrabhatla, Professor of Biology, Program Chair of Biology & Science, Penn State Harrisburg, Middletown, USA
DOI: 10.32474/CTBM.2021.02.000145
Abstract
Nutraceuticals have gained popularity for supplemental health benefits and for the ability to complement pharmaceuticals to prevent, delay, and manage chronic disease. The development of nutraceuticals involves the use of genome editing technology such as the newly developed CRISPR/Cas9 system, a novel and powerful method to knock out, knock-in, upregulate, and downregulate genes, including genes that can improve the nutritional profile of a plant as well as increase the production of nutraceuticals. Nutraceuticals, such as those implicated in aging, life expectancy, and chronic diseases can now be developed using CRISPR/Cas9. Establishing a CRISPR-based system to develop nutraceuticals and improve human health will benefit society.
Keywords: CRISPR/Cas9; Genome Editing; Nutritional Genomics; Nutraceuticals
Abbreviations: CRISPR: Clustered Regularly Interspaced Short Palindromic Repeats; Cas: CRISPR-Associated Protein; crRNA: CRISPR RNA; tracrRNA: Trans-Activating CRISPR RNA gRNA: guide RNA; sgRNA: Single Guide RNA; FAO: United Nations Food and Agricultural Organization; WHO: World Health Organization; FDA: Food and Drug Administration; DSHEA: Dietary Supplement Health and Education Act; GMP: Good Manufacturing Process; USDA: United States Department of Agriculture; GMO: genetically modified organism; EPA: Environmental Protection Agency; CR: caloric restrictions; IGF1R: insulin-like growth factor 1 receptor
Introduction
Overview of Nutraceuticals and Nutritional Genomics
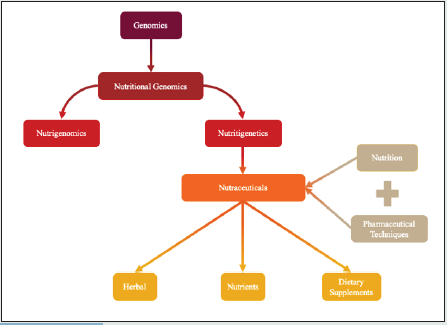
Stephen Defelice coined the term nutraceutical in 1989; it is defined as combining nutrition and pharmaceutical technology to create compounds that have beneficial effects on health and therapeutic uses [1,2]. Nutraceuticals can be divided into three categories: herbals, nutrients, and dietary supplements [3]. Nutraceuticals are derived using techniques and practices common in nutritional genomics, specifically in nutrigenetics. Nutritional genomics consists of nutrigenomics and nutrigenetics. Nutrigenomics studies the interaction of dietary components and the genome and changes that result, while nutrigenetics focuses on the development of nutraceuticals and analyzing differences at the gene level in response to dietary components [4]. A summarization regarding the classes of nutraceuticals can be found in Figure 1. Nutritional genomics is a relatively new division of genomics; it can be revolutionized with current and upcoming genome editing technology, including CRISPR/Cas9 genome editing.
CRISPR/Cas Genome Editing and Limitations
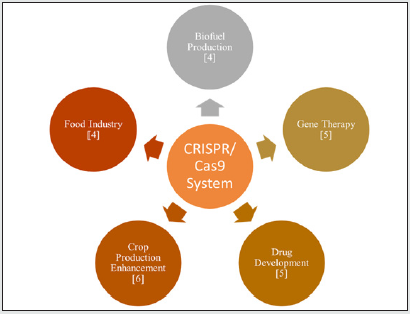
Genetic engineering has developed rapidly. The characteristic clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas) system has provided a novel and powerful method of gene editing; this method has many applications, which can be seen in Figure 2. The CRISPR/Cas’s genome-editing system is a part of the adaptive immune system that has developed in bacteria and archaea to defend against foreign agents [5]. There are two CRISPR/Cas’s system classes, with six types and multiple effectors [6,7]. Of the classes, the CRISPR/Cas II system (also known as the CRISPR/Cas9 system) is most used due to its simplicity and high efficacy [8]. This system contains the Cas9 protein, CRISPR RNA (crRNA), and trans-activating CRISPR RNA (tracrRNA). crRNA and tracrRNA form a duplex structure called the guide RNA (gRNA), which can be replaced by a single guide RNA (sgRNA) to form complementary base pairs with target DNA for cleavage at specific sites [9-10]. The sgRNA then guides the CRISPR/ Cas9 complex to the intended genomic location and then relies on one of the two endogenous DNA repair pathways, non-homologous end-joining (occurs more frequently) and homology-directed repair [11]. As a result, the system can easily be programmed and is a powerful tool for genomic engineering. As powerful of a tool the CRISPR/Cas9 system is for genomic engineering, there are limitations with this system. One of the significant limitations of CRISPR/Cas9 is the off-target effects [12-15]. Off-target effects result from the nonspecific activity of the Cas protein in non-target genomic location, and the off-target effects have a relatively high frequency with the application of the system [16,17].
There have been attempts to address this concern. Current attempts include engineered Cas9 variants and optimizing guide designs [18]. A variant of Cas9, Cas9 nickase, which induces singlestranded breaks, in combination with two sgRNAs, would produce a double-stranded break at the intended genomic location [19,20]. The paired nicking strategy considerably reduced the off-target activity in cell lines and produced the desired effect without giving up on-target cleavage efficiency [21]. Another potential strategy is to control the concentration of the Cas9-sgRNA complex; however, increasing the specificity by the reduction of transfected DNA also leads to on-target cleavage reduction [22]. While there are limitations and room for improvement, the CRISPR/Cas9 system has revolutionized genetic engineering.
Ethical Considerations with CRISPR/Cas9 should be written consistently as CRISPR/Cas9 and not Crispr/ Cas9
Biotechnology has rapidly expanded and has opened up new opportunities that did not seem plausible. One notable example of this advancement is the CRISPR/Cas9 system. This system allows the modification of the genome of organisms. However, despite these advances in biotechnology, there are many moral and ethical implications. An ethical consideration shared globally is that if CRISPR/Cas9 germline editing is permitted, it might lead to the return of eugenics [23]. Ethical concerns also arise for using CRISPR/Cas9 for non-therapeutic purposes such as enhancement of crops [24]. The CRISPR/Cas9 products impact society and humanity at large. The United Nations Food and Agriculture Organization, in 2016, had estimated that 795 million people in the world were undernourished; additionally, according to the World Health Organization, 2 billion people are unable to obtain necessary nutrients (Brokowski). There has been a significant amount of evidence that CRISPR/Cas9 could improve the nutritional content in foods [25]. While there are great benefits from the technology, concerns are raised about the fairness and affordability of these products [26].
Regulation on Nutraceuticals
Compared to the rapid growth and availability of nutraceuticals on the global market, the regulatory systems meant to oversee this market are evolving rather slowly [27]. Further complications arise from the vast differences in the Regulation of nutraceuticals between countries. However, most countries base their food use and safety regulations on the Codex Alimentarius, a collection of internationally recognized documents by both the United Nations Food and Agricultural Organization (FAO) and the World Health Organization (WHO), outlining the production and marking rules of foods and their derivatives [28]. As such, the Food and Drug Administration (FDA) in the US acknowledges and treats nutraceuticals differently than conventional foods and drugs. According to the Dietary Supplement Health and Education Act (DSHEA), the responsibility for the safety of nutraceuticals falls on the shoulders of manufacturers. Such safety includes but is not limited to having accurate information on nutraceutical labels that are reflective of the product being sold. Moreover, manufacturers and stakeholders do not need FDA approval before producing and selling food supplements and nutraceuticals [29]. An example of this is seen in the case of dietary supplements, a specific type of nutraceutical; manufacturers are free to use ingredients with different and controversial safety standards without formal FDA approval. Manufacturers must only demonstrate the history of use or other evidence that allows one to reasonably expect their safety when used according to the product label [30]. Furthermore, various sections of the FDA’s Modernization Act of 1997 allow health and nutrient content claims to be authorized based on statements from other federal authorities, such as the Academy of Sciences, after notifying the FDA four months prior to introducing the supplement to the market. A gray area in the past and ongoing regulations have led consumers and regulatory agencies to lose trust in the nutraceutical industry. The various health benefits associated with these products have been overshadowed by constant negative publicity due to lose Regulation, specifically labeling. From the contamination of nutraceuticals with drug products to dangerous side effects due to their lack of testing, the issues that plague the industry make it clear that the current Good Manufacturing Processes (GMP) implemented for conventional foods are inadequate. In terms of nutraceuticals, due to unsafe ingredients, the nonexistence of labeled ingredients, product purity, product potency, and product functionality, improvements are crucial to future success [31].
Regulation on CRISPR/Cas9 Genome Edited Products
Regulations of CRISPR/Cas9 Genome Edited Products (and other genetically engineered products) are outlined in the Coordinated Framework for the Regulation of Biotechnology, a proposal by the White House to ensure the safety of biotechnology products [32]. In 2016, the United States Department of Agriculture (USDA) announced that the first CRISPR/Cas9 engineered products, an anti-browning mushroom and waxy corn with starch composed solely of amylopectin, are exempt from the USDA’s genetically modified organism (GMO) regulations. These products did not meet the requirements for Regulation since no foreign DNA (transgene) from plant pests, such as bacteria or viruses, was inserted during editing. In addition, no traces of the CRISPR/ Cas9 system used during editing were left behind. They were the first CRISPR products approved for commercial use in the United States, avoiding regulatory obstacles [33,34]. In tandem with the USDA, the FDA and Environmental Protection Agency (EPA) are two other agencies that play a role in regulating genetically engineered products. Depending on the GMO in question, approval by all agencies may be necessary [35]. For instance, the EPA regulates pesticide safety, so GMOs that involve pesticides would be under the jurisdiction of the EPA. Any products that are meant to be consumed by humans most likely would fall under the jurisdiction of both the USDA and the FDA [36]. Moreover, US regulation of such products generally depends on its modified traits and intended use. The primary factors for consideration are efficacy and safety, whereas other factors, such as moral, cultural, and socioeconomic issues, bear significantly less weight in the product’s fate [37]. It is also important to note that the FDA concluded that there is little to no difference between genetically engineered and conventionally bred crops, suggesting that the agency has no further jurisdiction to mandate additional labeling [38]. Together, the USDA, FDA, and EPA continue to update the Coordinated Framework for the Regulation of Biotechnology and cover the vast array of genetically engineered products, ensuring product and consumer safety [39].
Current And Future Market Of CRISPR/Cas9 Concerning Nutraceuticals
Nutraceutical interest has increased in recent years due to potential health gains, both nutritional and therapeutic; the global market for nutraceuticals is massive and is worth approximately USD 117 billion; projections show that the market should continue to expand [40,41]. In 2017, US retail sales for herbal dietary supplements were over $8 billion [42,43]. A BCC report from 2018 also notes that by 2023, the global nutraceutical market should reach $336.1 billion [44]. In recent years, genome editing technologies, such as CRISPR/Cas9, have risen. CRISPR/Cas9 allows for easy manipulation of the DNA with a high efficiency which allows for diverse applications of the tool in various fields, including nutraceuticals and the development of nutraceuticals. The rapid expansion of the nutraceutical market and the growing demand for the development of nutraceuticals, combined with the capabilities of the CRISPR/Cas9 system of genome editing, open many doors. The market contains an active research field on nutraceuticals derived using CRISPR/Cas9 technology. These nutraceuticals have applications in overall health and wellness and support the structure and function of the body; examples include a delay in the aging process, increased life expectancy, and chronic disease prevention.
Body
Delay in Aging Process & Increase Life Expectancy
A current strategy for increasing life expectancy is delaying the onset of various age-related diseases [45,46]. As such, various studies have demonstrated that caloric restrictions (CR) without malnutrition is one of the most effective methods at increasing lifespan in species through the reduction of age-related diseases like Alzheimer’s disease, Parkinson’s disease, osteoporosis, obesity, diabetes mellitus, hypertension, cardiovascular diseases, and cancer [47,48]. However, due to the impracticality of CR, a recent study tested nutraceuticals as an alternative to CR by studying their effects on insulin-like growth factor 1 receptor (IGF1R) signaling and silent mating type information regulation two homolog 1 (SIRT1) activity. These are two signaling pathways that play a role in modulating longevity and healthspan: The study found that various nutraceuticals mimicked the effects of CR by modulating the IGF1R signaling and SIRT1 activity, thus promoting longevity [49]. Another study performed two human clinical trials in which nutraceuticals, specifically antioxidant supplements, were used to reduce urinary oxidative stress [50]. The first trial combined ThioMax, a mixture of α-lipoic acid and n-propyl gallate, with other antioxidants, minerals, vitamins, and omega-3 antioxidants to form YouthGuard reduced oxidative stress by 27%. The second trial used a plant extract (Thiogen), which contained tetramer to hexamer proanthocyanidin gallate-ester polymers and manganese to reduce oxidative stress by 38% [51] clinically. The study results, which cited previously published reports that demonstrated oxidative stress and specific nutritional deficiencies contribute to aging and age-related diseases, suggest that nutraceuticals, particularly various antioxidants and supplements, can delay the aging process and age-related diseases [52]. Other nutraceuticals identified to increase life expectancy include quercetin, fisetin, myricetin, epicatechin, luteolin, aspalathin, butein, DMC, stilbene, resveratrol, curcumin, berberine, lycopene, crocetin, corticosterone [53].
Chronic Disease Prevention
Nutraceuticals are often implicated in chronic disease
management and prevention, including but not limited to
neurodegenerative diseases, cardiovascular diseases, cancer, and
diabetes. Neurodegenerative diseases, such as Alzheimer’s disease,
which affects about 6.2 million elderly Americans, correlate with
oxidative stress [54]. Nutraceuticals that can act as antioxidants
can combat the oxidative stress associated with neurodegenerative
diseases. Such antioxidant nutraceuticals include curcumin, lutein,
lycopene, tumerin, and β-carotene; it is believed that consuming
antioxidant nutraceuticals may delay the development of
neurodegenerative diseases [55]. Nutraceuticals are also implicated
in the pathogenesis of Alzheimer’s, such as flavonoids, which were
found to inhibit neurological processes involved [56]. Flavonols
such as catechin, epicatechin, epigallocatechin, and epigallocatechin
gallate, have been implicated in cognitive improvement [57]. Notably,
Quercetin and Kaempferol are two abundant flavonoids found in
plants with antioxidant activity, cytoprotective effects, and other
positive effects on cognitive performance [58]. Nutraceuticals are
largely implicated in cardiovascular diseases, the leading cause of
death in the United States, presently as well; in fact, prevention and
management of cardiovascular diseases encompass nutraceuticals
including vitamins, minerals, antioxidants, dietary fibers, and
omega-3 polyunsaturated fatty acids [25,33]. Nutraceuticals,
especially polyphenols, assist in reducing cardiovascular diseases
through their ability to alter cell metabolism and signaling
[25,34,35]. Flavonoids in the form of flavones, falanones, and
flavanols have been found to block the angiotensin-converting
enzyme, cyclooxygenase enzymes and prevent platelet aggregation
protecting the vascular system [59]. Additionally, a bioflavonoid in
citrus fruits, hesperidin, is commonly used for venous insufficiency
and hemorrhoids [60].
However, consumption of flavonoids is inversely related to
coronary heart disease mortality and incidence of myocardial
infarction and is commonly consumed by the elderly to reduce the
chances of death from heart disease [25]. Phytosterols are also found
to have potential activity in reducing morbidity and mortality from
cardiovascular diseases, blocking cholesterol uptake, and assisting
its excretion [25]. Additionally, dietary fibers are also implicated
in having the ability to lower cholesterol [36]. Omega-3 fatty acids
affect plasma lipids and are largely implicated in cardiovascular
diseases [25,37]. Other lesser studied nutraceuticals implicated
in cardiovascular disease are anthocyanins, tannins such as
proanthocyanidins, tetrahydro-β-carbolines, stilbenes, dietary
indoleamines, serotonin, and melatonin [25]. Nutraceuticals are
also implicated in cancer prevention, management, and inhibition.
Globally, cancer is the leading cause of death, with almost 10 million
deaths in 2020 [38]. Lycopene, a carotenoid, is an antioxidant and
a singlet oxygen quencher that has been found to be protective
against cancer in the prostate, testes, and skin, where it is
concentrated; it also decreases oxidative stress and DNA damage
[25,39,40]. Antioxidant nutraceuticals are primarily implicated
in cancer, for example, β-carotene. Flavonoids such as isoflavones
act as antioxidants and have chemopreventive properties [61].
Furthermore, studies are reporting saponins to have antimutagenic
and anticarcinogenic activity, while tannins are found to be harmful
free radical scavengers and detoxify carcinogens [25]. Pectin has
been implicated in prostate cancer metastasis by blocking cancer
cells from adhering to other cells, notably [25,42]. Other lesser
studied nutraceuticals implicated in cancer include daidzein,
biochanin, isoflavones, phenolic compounds like gallic acid,
glucosinolates, cysteine, glutathione, selenium, vitamin E, vitamin C,
and genistein; additionally, phytochemicals and phytoestrogens are currently being researched for their anticarcinogenic potential [43].
Regarding diabetes, which affects 34.2 million in the United States,
or 10.5% of the population, nutraceuticals can make the quality of
life better [44]. The most studied nutraceuticals for diabetes are
isoflavones, and they have been associated with a lower incidence
and mortality rate for type II diabetes [45]. In conjunction, Omega-3
fatty acids are implicated in glucose tolerance reduction in those
with diabetes; Lipoic acid has been used in treating diabetic
neuropathy and can protect diabetic patients from complications
or comorbidities [25,46]. Dietary fibers have also been used for
glucose control in diabetics [25]. Though neurodegenerative
diseases, cardiovascular disease, cancer, and diabetes are not an
exhaustive list of chronic diseases, they highlight the potential of
nutraceuticals in the prevention, management, treatment, and
delaying chronic disease with much ground in research still to
cover to understand the potential of nutraceuticals fully.
The Implication of CRISPR/Cas9 on Nutraceuticals
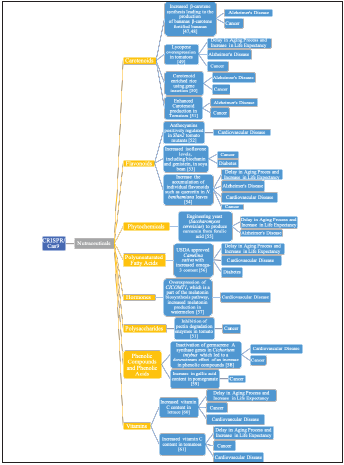
Nutraceuticals may be beneficial to many aspects of health, leading to the demand for the development and production of nutraceuticals. One such method of nutraceutical development and production is the CRISPR/Cas9 system. The CRISPR/Cas9 system can be used as a tool to develop nutraceuticals and enhance the production of nutraceuticals in medicinal plants. For medicinal plants and nutraceuticals, the CRISPR/Cas9 genome editing system can manipulate the phytochemical profile to achieve one more desirable. (Figure 3) summarizes the recent development of the production of nutraceuticals using the CRISPR/Cas9 system. The CRISPR/Cas9 system can be exploited in developing and producing nutraceuticals, as shown in figure 3. More research on how to use the full potential of this system to develop a wide array of nutraceuticals efficiently is a current active field of research.
Conclusion
Nutraceuticals have a role in many aspects of health: the aging process, life expectancy, chronic disease prevention/management, and delaying the onset of chronic disease. Many of these nutraceuticals, in recent years, have been developed using CRISPR/ Cas9 systems that increase the nutraceutical concentration in the medicinal plant from which it is derived. These CRISPR/Cas9- developed nutraceuticals are at the forefront of nutraceutical development and CRISPR/Cas9 research. They are slowly making their way onto the global market with the implementation of Regulation on nutraceuticals and CRISPR products and considering the ethics of using CRISPR.
Acknowledgments
The authors would like to acknowledge the School of Science, Engineering, and Technology at Penn State Harrisburg. All authors were equally contributed.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this review.
References
- Kalra EK (2003) Nutraceutical-definition and introduction. Aaps Pharmsci 5(3): 27-28.
- Ghani U, Naeem M, Rafeeq H, Imtiaz U, Amjad A, et al. (2019) A Novel Approach towards Nutraceuticals and Biomedical Applications. Sch Int J Biochem 2(10): 245- 252.
- Subbiah MR (2007) Nutrigenetics and nutraceuticals: the next wave riding on personalized medicine. Translational Research 149(2): 55-61.
- Nidhi S, Anand U, Oleksak P, Tripathi P, Lal JA, et al. (2021) Novel CRISPR–Cas Systems: An Updated Review of the Current Achievements, Applications, and Future Research Perspectives. International Journal of Molecular Sciences 22(7): 3327.
- Djekoun A (2021) Therapeutic and diagnostic relevance of Crispr technology. Biomedicine & Pharmacotherapy 138: 111487.
- Eş I, Gavahian M, Marti-Quijal FJ, Lorenzo JM, Khaneghah AM, et al. (2019) The application of the CRISPR-Cas9 genome editing machinery in food and agricultural science: Current status, future perspectives, and associated challenges. Biotechnology advances 37(3): 410-421.
- Liu C, Zhang L, Liu H, Cheng K (2017) Delivery strategies of the CRISPR-Cas9 gene-editing system for therapeutic applications. Journal of controlled release 266: 17-26.
- Barman A, Deb B, Chakraborty S (2020) A glance at genome editing with CRISPR–Cas9 technology. Current genetics 66(3): 447-462.
- Arora L, Narula A (2017) Gene editing and crop improvement using CRISPR-Cas9 system. Frontiers in plant science 8: 1932.
- Martinez-Lage M, Puig-Serra P, Menendez P, Torres-Ruiz R, Rodriguez-Perales S (2018) CRISPR/Cas9 for cancer therapy: hopes and challenges. Biomedicines 6(4): 105.
- Wang H, La Russa M, Qi LS (2016) CRISPR/Cas9 in genome editing and beyond. Annual review of biochemistry 85: 227-264.
- Uddin F, Rudin CM, Sen T (2020) CRISPR gene therapy: applications, limitations, and implications for the future. Frontiers in Oncology 10: 1387.
- Gupta RM, Musunuru K (2014) Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. The Journal of clinical investigation 124(10): 4154-4161.
- Plaza Reyes A, Lanner F (2017) Towards a CRISPR view of early human development: applications, limitations and ethical concerns of genome editing in human embryos. Development 144(1): 3-7.
- Yang Y, Xu J, Ge S, Lai L (2021) CRISPR/Cas: advances, limitations, and applications for precision cancer research. Frontiers in Medicine p. 8.
- Zhang XH, Tee LY, Wang XG, Huang QS, Yang SH (2015) Off-target effects in CRISPR/Cas9-mediated genome engineering. Molecular Therapy-Nucleic Acids, 4(11): e264.
- Brokowski C, Adli M (2019) CRISPR ethics: moral considerations for applications of a powerful tool. Journal of molecular biology 431(1): 88-101.
- Santini A, Cammarata SM, Capone G, Ianaro A, Tenore GC, et al. (2018). Nutraceuticals: opening the debate for a regulatory framework. British journal of clinical pharmacology 84(4): 659-672.
- Wrick K L (2005) Regulation of quality and quality issues worldwide. In Hasler C. M. (Ed.) Regulation of functional foods and nutraceuticals: A global perspective Blackwell Publishing, USA pp. 55.
- Joseph, Joy (2005) Regulation of quality and quality issues worldwide. Hasler CM (Eds.), Regulation of functional foods and nutraceuticals: A global perspective, Blackwell Publishing, USA pp. 55.
- Waltz E (2016) CRISPR-edited crops free to enter market, skip regulation. Nat Biotechnol 34(6): 582.
- Globus R, Qimron U (2018) A technological and regulatory outlook on CRISPR crop editing. Journal of cellular biochemistry 119(2): 1291-1298.
- El-Mounadi K, Morales-Floriano ML, Garcia-Ruiz H (2020) Principles, applications, and biosafety of plant genome editing using CRISPR-Cas9. Frontiers in plant science 11: 56.
- Sachdeva V, Roy A, Bharadvaja N (2020) Current prospects of nutraceuticals: A review. Current pharmaceutical biotechnology 21(10): 884-896.
- Nasri H, Baradaran A, Shirzad H, Rafieian-Kopaei M (2014) New concepts in nutraceuticals as alternative for pharmaceuticals. International journal of preventive medicine 5(12): 1487-1499.
- Williamson EM, Liu X, Izzo, AA (2020) Trends in use, pharmacology, and clinical applications of emerging herbal nutraceuticals. British Journal of Pharmacology 177(6): 1227-1240.
- Smith T, Kawa K, Eckl V, Morton C, Stredney R (2018) Herbal supplement sales in US increased 8.5% in 2017 topping $8 billion. HerbalGram 119: 62-71.
- Nutraceuticals: Global markets to 2023. BCC Research.
- Villeponteau B, Cockrell R, Feng J (2000) Nutraceutical interventions may delay aging and the age-related diseases. Experimental gerontology 35(9-10): 1405-1417.
- Pavlović I, Khateb S, Milisav I, Mahajna J (2020) Nutraceuticals for Promoting Longevity. Current Nutraceuticals 1(1): 18-32.
- Alzheimer's Association. (2019). 2019 Alzheimer's disease facts and figures. Alzheimer's & dementia 15(3): 321-387.
- Mecocci P, Tinarelli C, Schulz RJ, Polidori MC (2014) Nutraceuticals in cognitive impairment and Alzheimer's disease. Frontiers in pharmacology 5: 147.
- Center for Disease Control and Prevention (2021) Heart Disease Facts.
- Asgary S, Sahebkar A, Afshani MR, Keshvari M Haghjooyjavanmard S (2014) Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytotherapy research: PTR 28(2): 193-199.
- Asgary S, Rastqar A, Keshvari M (2018) Functional Food and Cardiovascular Disease Prevention and Treatment: A Review. Journal of the American College of Nutrition 37(5): 429-455.
- Abdul-Hamid A, Luan YS (2000) Functional properties of dietary fibre prepared from defatted rice bran. Food chemistry 68(1): 15-19.
- Sidhu KS (2003) Health benefits and potential risks related to consumption of fish or fish oil. Regulatory toxicology and pharmacology: RTP 38(3): 336-344.
- World Health Organization (2021) Cancer.
- Willis MS, Wians FH (2003) The role of nutrition in preventing prostate cancer: a review of the proposed mechanism of action of various dietary substances. Clinica chimica acta; international journal of clinical chemistry 330(1-2): 57-83.
- Shirzad H, Kiani M, Shirzad M (2013) Impacts of tomato extract on the mice fibrosarcoma cells. Journal of Herbmed Pharmacology 2(1): 13-16.
- Thomasset SC, Berry DP, Garcea G, Marczylo T., Steward WP, et al. (2007) Dietary polyphenolic phytochemicals--promising cancer chemopreventive agents in humans? A review of their clinical properties. International Journal of cancer 120(3): 451-458.
- Nasri H, Sahinfard N, Rafieian M, Rafieian S, Shirzad M, et al. (2014) Turmeric: A spice with multifunctional medicinal properties. Journal of HerbMed Pharmacology 3(1):5-8.
- Limer JL, Speirs V (2004) Phyto-oestrogens and breast cancer chemoprevention. Breast cancer research BCR 6(3): 119-127.
- Center for Disease Control and Prevention (2021) Diabetes Data and Statistics.
- Tavafi M (2013) Diabetic nephropathy and antioxidants. Journal of nephropathology 2(1): 20-27.
- Coleman MD, Eason RC, Bailey CJ (2001) The therapeutic use of lipoic acid in diabetes: a current perspective. Environmental toxicology and pharmacology 10(4): 167-172.
- Kaur N, Alok A, Shivani Kumar P, Kaur N, et al. (2020) CRISPR/Cas9 directed editing of lycopene epsilon-cyclase modulates metabolic flux for β-carotene biosynthesis in banana fruit. Metabolic engineering 59: 76-86.
- Dey A (2021) CRISPR/Cas’s genome editing to optimize pharmacologically active plant natural products. Pharmacological research 164: 105359.
- Li X, Wang Y, Chen S, Tian H, Fu D, et al. (2018) Lycopene is enriched in tomato fruit by CRISPR/Cas9-mediated multiplex genome editing. Frontiers in plant science 9: 559.
- Dong OX, Yu S, Jain R, Zhang N, Duong PQ, et al. (2020) Marker-free carotenoid-enriched rice generated through targeted gene insertion using CRISPR-Cas9. Nature communications 11(1): 1-10.
- Wang D, Samsulrizal NH, Yan C, Allcock NS, Craigon, et al. (2019) Characterization of CRISPR mutants targeting genes modulating pectin degradation in ripening tomato. Plant physiology 179(2): 544-557.
- Zhi J, Liu X, Li D, Huang Y, Yan S, et al. (2020) CRISPR/Cas9-mediated SlAN2 mutants reveal various regulatory models of anthocyanin biosynthesis in tomato plant. Plant cell reports 39(6): 799-809.
- Zhang P, Du H, Wang J, Pu Y, Yang C, et al. (2020) Multiplex CRISPR/Cas9‐mediated metabolic engineering increases soya bean isoflavone content and resistance to soya bean mosaic virus. Plant biotechnology journal 18(6): 1384-1395.
- Selma S, Sanmartín N, Espinosa-Ruiz A, Gianoglio S, Lopez-Gresa MP, et al. (2021) Custom-made design of metabolite composition in N. benthamiana leaves using CRISPR activators. bioRxiv.
- Costa JMR, Gomes DFC, Rodrigues JLLC, Rodrigues LR (2019) CRISPR-Cas9 based strategy to engineer Saccharomyces cerevisiae towards the production of curcumin from ferulic acid.
- Waltz E (2018) With a free pass, CRISPR-edited plants reach market in record time. Nat Biotechnol 36(1): 6-7.
- Chang J, Guo Y, Yan J, Zhang Z, Yuan L, et al. (2021) The role of watermelon caffeic acid O-methyltransferase (ClCOMT1) in melatonin biosynthesis and abiotic stress tolerance. Horticulture research 8(1): 1-12.
- Cankar K, Bundock P, Sevenier R, Häkkinen ST, Hakkert JC, et al. (2021) Inactivation of the germacrene A synthase genes by CRISPR/Cas9 eliminates the biosynthesis of sesquiterpene lactones in Cichorium intybus L. Plant Biotechnology Journal 19(12): 2442-2453.
- Chang L, Wu S, Tian L (2019) Effective genome editing and identification of a regiospecific gallic acid 4-O-glycosyltransferase in pomegranate (Punica granatum L.). Horticulture research 6(1): 1-15.
- Li T, Yang X, Yu Y, Si X, Zhai X, et al. (2018) Domestication of wild tomato is accelerated by genome editing. Nature biotechnology 36(12): 1160-1163.
- Zhang H, Si X, Ji X, Fan R, Liu J, Chen K, et al. (2018) Genome editing of upstream open reading frames enables translational control in plants. Nature Biotechnology 36(9): 894-898.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...