Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1725
Research Article(ISSN: 2641-1725) 
Quetiapine Poisoning – Epidemiology, Toxicokinetics and Review of the Literature Volume 5 - Issue 5
Langberg C1, Hadley CL2, Midtrevold M3, Edvardsen HME4, Molden E5, Shafiei M6 and Jacobsen D1,2,3*
- 1Institue of Clinical Medicine, University of Oslo, Norway
- 2Department of Acute Medicine at the Oslo University Hospital, Norway
- 3Norwegian Poisons Information Centre, Norway
- 4Department of Forensic Sciences at the Oslo University Hospital, Norway
- 5Diakonhjemmet Hospital, Oslo, Norway
- 6Department of Surgery and Anaesthesiology at Skien Hospital, Telemark, Norway
Received: July 27, 2021 Published: August 4, 2021
*Corresponding author: Jacobsen D, Department of Acute Medicine at the Oslo University Hospital, Norway
DOI: 10.32474/LOJMS.2021.05.000224
Abstract
Background: With increasing use and availability of Quetiapine (Q) the number of self-poisonings has increased. The toxic effects of Q may be lethal and an increased knowledge of these poisonings may improve the outcome in these patients.
Methods: Two cases of Q poisoning are presented, one with toxicokinetic data of both Q and NQ, as well as a literature review of Q poisoning. Epidemiological data on Q poisoning in Norway and the frequency of these poisonings in the forensic reports are presented in the supplementary material.
Results: The two cases of severe Q poisoning, of whom one was fatal, illustrated the cardiovascular complications and the convulsions also found in the literature review. The toxicokinetic data indicated a biphasic elimination of Q and a persisting high level of the metabolite N-desalkylquetiapine (NQ) even after 80 hours. Q was a toxic agent in 1-2% of the inquiries to the National Poisons Information Center and was detected in 7% of the poisoning fatalities.
Conclusion: As Q poisonings have increased, clinicians need to be aware of the cardiovascular and neurological complications of these poisonings. The toxicokinetic data may indicate delayed gastric emptying in severe poisoning. This may be due to anticholinergic effects, and thus gastric lavage with installation of active charcoal should be considered even later than two hours after ingestion. Patients with suspected severe Q poisoning should be admitted to an ICU as their condition may deteriorate rapidly.
Introduction
Quetiapine (Q) is an atypical antipsychotic drug that is increasingly used as first line treatment for schizophrenia, manicand severe depressive episodes of bipolar disorder [1,2]. In Norway, Q is used as first line treatment for these disorders and during the period from 2009 until 2019, the number of users has increased from 2.3/1000 inhabitants to 12.9/1000 inhabitants [3]. However, there is also an increasing use of Q as a hypnotic, although this use is unapproved [4]. Similar to other atypical antipsychotics, Q is known to bind to numerous serotonergic, dopaminergic, adrenergic, histaminergic and muscarinic receptors with different affinities. Compared to the other atypical antipsychotics, Q has less ability to give extrapyramidal features [5]. With increased availability of the drug, the number of overdoses has increased [6]. Although several case reports describe the clinical course of Q overdoses [6-46], few are on large overdoses and few to none report sequelae and data on the toxicokinetics of the major active metabolite N-desalkylquetiapine (NQ). The aim of this study was to review the literature on the clinical presentation, complications and toxicokinetics of Q poisoning. Two cases are presented to illustrate the complexity of severe Q poisoning. We also studied the number of inquiries on Q to the National Poisons Information Center and the number of fatal poisonings where Q was detected in the forensic analyses (Supplementary material).
Materials and Methods
Case reports
In both cases Q and NQ were measured by a valid LC MS/MS (Liquid chromatography with tandem mass spectrometry) method developed for routine therapeutic drug monitoring analyses at the Centre for Psychopharmacology, Diakonhjemmet Hospital, Norway. The retention times were 2.17 min for NQ and 2.39 for Q (internal standard). Detection was performed at the following mass transitions: m/z 296 - 210 for NQ, and m/z 384-253 for quetiapine. The lower limit of quantification was 0.020 and 0.025μmol/L for Q and NQ respectively, with imprecision and inaccuracy parameters less than 5%. SI-conversion factors for Q and NQ (μmol/L-mg/L) are 0.384 and 0.296, respectively. Informed consent for presenting the case in the literature as a case report was obtained from patient 1 and relatives in case 2.
Literature Search Strategy
A systematic review of the literature was conducted to evaluate reported information on quetiapine overdoses and to uncover the toxicokinetics of quetiapine. An extensive literature search was performed and included the following databases: Ovid platforms PubMed, EMBASE, Cochrane Library, SveMed+, Toxnet, The Norwegian Electronic Health Library (Helsebiblioteket.no), The Norwegian Medicines Agency and its NoMA medicine database (Statens legemiddelverk) with the Norwegian SPC and Electronic Medicine Compendium (EMC) with the British SmPC. We included case reports, letters to the editor, abstracts and retrospective studies. The search strategy was limited to publications less than 10 years old in English and Scandinavian languages and excluded animal studies. The full search parameters are included in the Supplementary material.
Inclusion and Exclusion Criteria
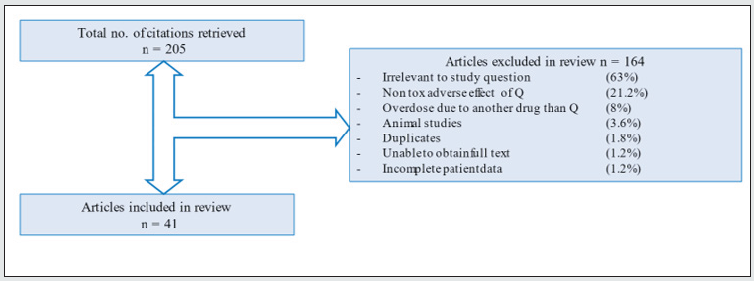
We included studies on Q overdoses, or other toxic and severe adverse effects of Q use in humans. We excluded reports that did not report Q as the main or contributing agent in the poisoning, and reports where clinical- or toxicokinetic data on quetiapine were missing (Figure 1).
Poisons Center and Forensic Data
This information was based on their annual reports (see Supplementary material).
Statistics
Median ranges were calculated by including medians from case series and big cohort studies, and a calculated median combining all single case studies. Due to issues obtaining full access to statistics from articles included.
Results
Case 1
A middle-aged woman, with a history of a bipolar disorder was brought to hospital with a presumed Q overdose. The patient was found somnolent in her home along with empty packets of quetiapine 100mg tablets estimated to a total of 30g. The patient was last seen nine hours before admission on leave from a psychiatric ward. On admission, the patient was somnolent with Glasgow Coma Scale (GCS) 10. Her blood pressure was 133/80 mmHg, heart rate 115 bpm and respiratory rate 12 per minute. The pupils were miotic and responded poorly to light. Her skin was warm and dry, but she was hypothermic (35.2°C). She had urinary retention with 500 mL of urine obtained after catheterisation. Her Electrocardiogram (ECG) was normal with QTc 478ms. On admission, the arterial blood gas showed a pH 7.38, pO2 4.63 kPa, pCO2 4.6 kPa, and base excess -4.3 mmol/L. Her blood tested negative for ethanol and paracetamol. CK was elevated at 4600 U/L, but creatinine was normal. Glucose was 6.2 mmol/L, electrolytes and serum osmolality were all within normal range. Gastric lavage with subsequent installation of activated charcoal was not performed as suspected time since ingestion exceeded two hours.
Ten hours after admission, the patient developed generalized seizures treated with benzodiazepines, but the seizures progressed to status epilepticus. The patient developed acute respiratory collapse, hypotension (90/40 mm Hg) and increasing bradycardia with wide QRS complexes. She was intubated and received successful Cardiopulmonary Resuscitation (CPR). The ECG showed widened QRS complexes (150 ms), sinus tachycardia (120/ min), 1st degree AV-block, LBBB configurations and prolonged QTc of 510ms. After return of spontaneous circulation (ROSC), she was given infusions with norepinephrine, sodium valproate, benzodiazepines and fentanyl. A chest X-ray showed bilateral lung consolidations, probably caused by aspiration. Clinically, she had developed Acute Respiratory Distress Syndrome (ARDS). Blood tests revealed severe metabolic acidosis with pH 6.7, lactate 18 mmol/L and signs of rhabdomyolysis (CK 4670 U/L) without renal failure. Suspected sepsis was treated with broad-spectrum antibiotics. Echocardiography showed no structural abnormalities. The respiratory support was gradually increased to maintain satisfactory oxygenation as her condition deteriorated. On day nine her ARDS was so severe that Extracorporeal Membrane Oxygenation (ECMO) treatment was discussed. However, this was not necessary as her condition gradually improved. Repeated Computed Tomography (CT) scans of the brain were normal.
On day 26 she was successfully extubated and rehabilitation was initiated. 39 days after admission, of which 28 days in an ICU, the patient was stable and transferred to a psychiatric ward for further treatment.
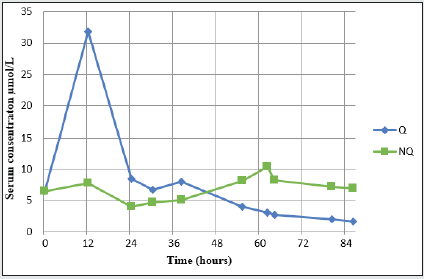
The concentration of Q peaked 12 hours after admission at 31.8 μmol/L before declining. The elimination was rapid initially, and during the 12-30 hour samples, the apparent T1/2 was estimated to be 8 hours. Over the next day`s (30-86 hours) Q elimination was slower with an apparent T1/2 of 75 hours. As Q declined, its metabolite NQ increased reaching a peak concentration of 10.2μmol/L 62 hours after admission (Figure 2).
Case 2
A 20-year-old woman with a history of depression was brought to hospital with a presumed self-poisoning. She was found unconscious and cyanotic in her home with empty packages of sertraline, hydroxyzinhydrokloride and Q. Paramedics initiated Advanced Cardiopulmonary Resuscitation (ACPR) and administered 5 mg of epinephrine. On admission, she was comatose, intubated, hypotensive (43/28 mmHg), and had a pulse frequency varying between 74-105/min. Gastric lavage was performed on admission with no tablets found. Activated charcoal was administered. Arterial blood gas showed a severe combined metabolic and respiratory acidosis with pH 6.95, pO2 4.3 kPa, pCO2 11.2 kPa, HCO3- 19 mmol/L, base excess -13 mmol/L, lactate 14 mmol/L, haemoglobin (Hb) 9.7 g/dL, Na+ 140 mmol/L, K+ 4.5 mmol/L. ACPR was again required. A total of 7 mg epinephrine was administered. NaHCO3 was also given i.v. The patient had rapid transitory return of circulation (ROSC) in the ER. She was bleeding from the intubation tube and Hb level fell to 6.5 g/dL. She was given two units of erythrocytes in Salineadenine- glucose (SAG) and crystalloids.
ECG showed widened QRS-complexes with branch block patterns. CT scan of the brain showed signs of marked oedema with indistinguishable surface relief and lack of distinction between white and grey matter. CT scans findings were consistent with massive lung haemorrhage bilaterally and in the small intestines. Despite initiated resuscitation and treatment, the patient’s condition deteriorated. ACRP was discontinued 1.5h after admission and the patient was declared dead. Blood taken on admission showed S-sertraline 1.229μmol/L (0.020-0.250 μmol/L) and S-quetiapine 4.753μmol/L (0.050-0.700μmol/L), both high concentrations compatible with a severe overdose.
Review of the Literature
Mortality
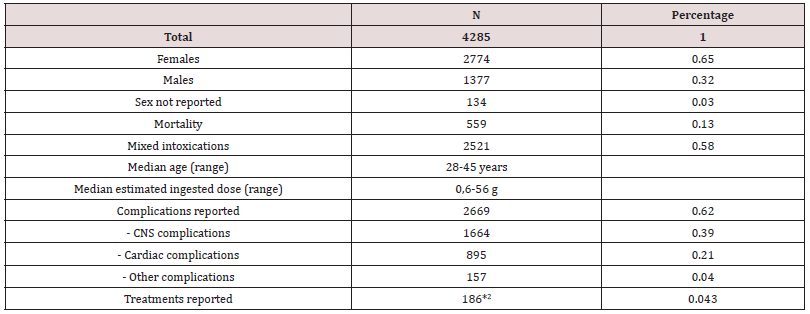
The literature review included 4285 patients with Q poisoning, 2774 (65%) women and 1377 (32%) males (Table 1). Median agerange from 28 to 45 years Dose ingested ranged from 0.6-56 g. A total of 559 deaths (13%) were reported. Fifty-eight percent of the reported Q intoxications were mixed or suspected mixed drug intoxications.
1Includes suspected mixed intoxications.
2Includes several treatments in the same cases. Not all cases report any treatment.
Administration and Formulation
In 99.7% of the cases, Q was taken by ingestion. Q has two formulations, an extended release (XR) formula (in 62%) and an Immediate Release (IR) formula (in 38% of the cases).
1Other ECG changes e.g. left and right bundle brach block and widened QRS-complex.
2Includes difficulty breathing, desaturation and respiratory collapse.
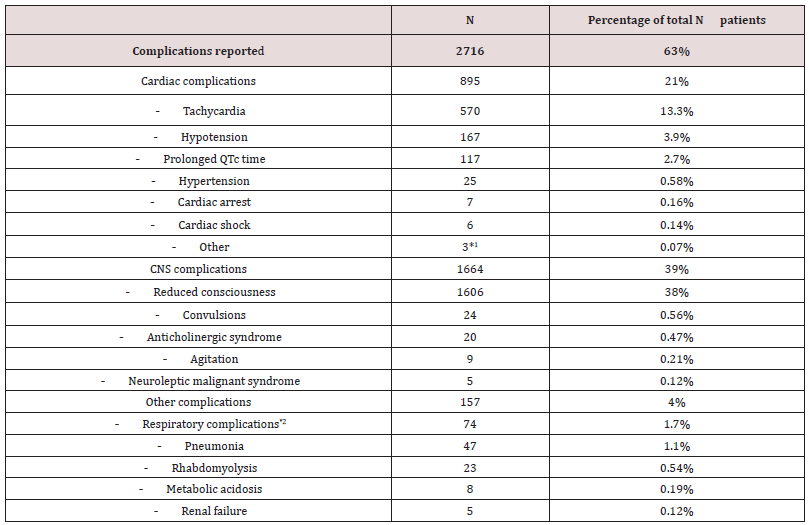
Cardiac complications were reported 895 times (21%), (Table 2). Most common were tachycardia (n=570;13%), hypotension (n=167;3.9%) and prolonged QT time (n=117; 2.7%). Complications related to the central nervous system (CNS) are listed in table 2. The most common complication was reduced consciousness in 1606 cases (38%) whereas convulsions were only reported in 24 (0.56%). Other complications (4%) were rare.
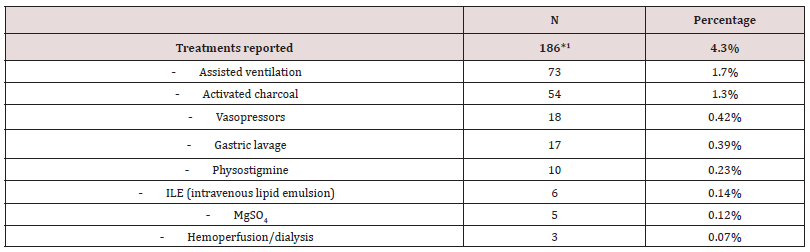
Treatment
A total of 186 treatment interventions were reported (Table 3), of which assisted ventilation was the most frequent (1.7%). Note that activated charcoal was only given in 1.3% and gastric lavage only in 0.39% of the cases.
1Includes several treatments in the same case. Not all cases reported any treatment.
Data from the National Poisons Information Center (NPIC) and the Forensic data are given in Supplementary material
Only 1-2% of the inquiries to the NIPC were about Q which was found in 31 (7%) of the 468 overdose deaths in Norway (2016- 2017).
Discussion
Toxicokinetics
The absorption of Q is reported to be rapid and 100% [1] and marginally affected by administration of food [47]. Q is extensively metabolised following oral administration according to the SPC [1]. Quetiapine is reported to cause constipation and have anticholinergic effects due to its pharmacodynamic antagonistic properties on muscarinic M1 receptor. A delayed onset of toxicity and prolongation of symptoms has also been associated with different pharmacokinetics between the two formulations of Q, immediate release IR and extended release XR. The study from Taylor et al. comparing the overdoses with the two different formulations of Q reported an association between the XR formulation and a delayed onset of toxicity compared to IR [41]. This may be due to a possible bezoar formation from the tablets in the ventricle forming a gel in Q overdoses that contributes to a delayed absorption [34]. However, the serum max concentration (Tmax) is reported to be 1-2 hours with IR and 6 hours with XR [48,49].
Case 1 illustrates the elimination of Q and NQ from a large Q overdose (Figure 2). The Q levels increased rapidly and peaked 12 hours after admission at 31.8 μmol/L. The elimination was rapid in the first 12-30 hours with an estimated T1/2 of 8 h. During the next 30-86 hours, the elimination was slower with an estimated T1/2 of 75 h. The decrease of Q in serum the first 12-30 hours may indicate a first phase, a T1/2- alfa, where distribution of Q is taking place. The second phase, a T1/2-beta, lasting the next 30-86 hours, usually describes an elimination phase. This is most often of 1st order kinetic, but the T1/2 was here prolonged more than expected and other mechanisms may thus play a role. Giuntoli, et al. [24], discusses similar models of Q toxicokinetics in overdoses up to 24g, although the study also discusses contradicting evidence in the literature that may indicate altered pharmacokinetics. Papazisis, et al. [29], also reported biphasic Q toxicokinetics. This two-phase elimination curve combined with a prolonged T1/2 has also been described by Pollack, et al. [50] and may not be explained by neither 0. nor 1. order kinetics. This may indicate biphasic kinetics occurring during large Q poisoning and a prolonged T1/2 in overdoses. Rauber et al. [34] have described quetiapine tablets in gastric lavage from 2.25-13 hours after ingestion. Prolonged T1/2 and the biphasic kinetics of Q in overdose may be due to anticholinergic activity with reduced GI motility and prolonged Q absorption, with absorption and metabolism continuing over an extended period. Most case reports with lower quetiapine doses describe symptom remission within 48 hours. This is in accordance with the rapid elimination/distribution of Q observed the first 24 hours in our patient. According to the SPC of Seroquel, the elimination halflives of Q and NQ are approximately 7 and 12 hours, respectively [1,2]. These half-lives seem to be underestimated for an overdose situation. Both Eyer et al. and Hantson, et al. [21, 25] estimated T1/2 of Q to be in the range 16-62 hours. If so, gastric lavage may still be recommended in large overdoses of Q even if time since ingestion exceeds two hours.
NQ elimination during an overdose has not yet been reported in the literature. In our case 1, the levels of NQ increased after admission and peaked at 10.3 μmol/L 62 hours after admission (Figure 2). However, the levels remained high even after 80 hours. This does not correlate with experimental work in animal models where elimination concentrations within the therapeutic range of Q and NQ correlated well (5). As seen in Figure 2, there was an increase in the serum concentration of NQ when the Q concentration was decreasing. This may indicate a saturation situation where more NQ accumulates and exceeds the capacity to eliminate. NQ is metabolized and eliminated through CYP3A4 similar to Q [1]. A prolonged absorption of Q may be due to anticholinergic effects. This may explain why NQ levels are increasing and remain high for a long time period while Q levels are decreasing.
During the last 10 years, 58% of the quetiapine overdoses were overdoses with multiple drugs (Table 1). The co-ingestions reported were mainly benzodiazepines, antidepressants (SSRI) and ethanol but also drugs as antibiotics and antiviral drugs have been implicated due to CYP3A4 inhibition [14,25]. The FDA advice is to avoid alcohol consumption with quetiapine [47]. Clinicians should be aware of possible drug interactions through CYP3A4 inhibition in Q overdoses, which may affect the toxicokinetics of Q.
Complications
Pharmacodynamically, Q acts as a serotonin 5HT2A, dopamine D2, alpha-1-adrenergic and histamine H1 antagonist, and a serotonin 5HT1A partial agonist, and NQ as an serotonin receptor 5HT1A agonist and histamine H1, D2-, 5HT2A, alpha-adrenergic 1A and 2 antagonist, and a norepinephrine transporter inhibitor [5]. The most common complications reported were reduced consciousness, tachycardia, hypotension, and prolonged QTc-time. In comparison to other atypical antipsychotics, Q is reported to cause less extrapyramidal adverse effects. However, in one retrospective poison center data study, coma, respiratory depression requiring intubation, hypotension and death were more likely to occur after Q overdose compared to all other antipsychotics as a group [51]. The clinical presentation of our two cases gives an insight into different courses and toxicokinetic aspects of severe Q poisoning. Case 1 showed a delayed presentation of severe symptoms and a prolonged T1/2 of Q. It also showed serum concentrations of NQ with a delayed peak in concentration and an unusual elimination. Case 2 showed complications of Q poisoning where interventions were unsuccessful. The critical illness of our two patients could be an example of publication bias towards the most severe-and challenging-cases which may explain the relatively high mortality reported on Q poisonings in the literature.
The clinical course with rapid deterioration of the patient’s condition after admission in the first case, underlines the importance of admitting patients with a presumed large quetiapine overdoses to an Intensive Care Unit (ICU) even when a patient is stable on admission. This is also emphasised in the literature where delayed onset of toxicity after Q overdose have occurred in several studies [11, 35, 41]. A study from Taylor, et al. [41] also observed a longer delayed onset of toxicity in the extended-release formulation, thus recommending monitoring the patients for symptoms up to 12 hours post overdose to rule out significant poisoning.
The reported toxicity of Q is very variable and thus it may be difficult to predict the severity of the poisonings. Studies report that the severity of the Q overdose does not seem to be associated with a high serum concentration nor with the reported ingested dose [10,21,29,52]. Fatal cases have been reported to occur after therapeutic doses as well as massive ingestions [31,53]. Deaths have been reported at Q concentrations lower than 36 mg [54], and patients have survived acute Q overdoses of up to 36g [10, 46]. On the other hand, a study from Isbister et al. on predicting intubation, duration of ventilation, cardiac monitoring and the effect on activated charcoal, reported a 3g limit of ingested Q to have increased risk of CNS affection, coma and hypotension [55]. They also estimated a probability of intubation after ingested amounts of Q: 10% after 2 grams, 22% after 5 grams, 37% after 10 grams, 55% after 20 grams. Peredy et.al observed an increased risk of severe Q poisoning when associated with other psychotic drugs such as benzodiazepines and antidepressants [31]. In the literature review, most Q overdoses were mixed intoxications with co-ingestions. Reduced consciousness was observed in both patients and this was the most common complication reported in the literature. Both patients were hypothermic and acidotic although only very few have reported metabolic acidosis in the literature. Both patients developed hypotension and showed widening of the QRS complex, bundle branch blocks and the first patient had significantly prolonged QTc-time. These cardiac complications are known from the literature [8,26,45].
Management
It is reported that hypotension from Q poisoning responds poorly to fluids or epinephrine, but responds to norepinephrine [56,57]. Worsening of hypotension in Q overdose after administration of epinephrine may likely be due to high beta-2 adrenoceptor mediated relaxation in vascular smooth muscle and skeletal muscle beds resulting in an overall decrease in systemic vascular resistance in the setting of induced alfa-adrenal receptor antagonism [42]. The SPC, SmPC and the NPIC, do not recommend the use of epinephrine in treatment of hypotension in quetiapine overdoses [1,56,57]. Hypotension mediated by alpha-1-adrenergic receptor antagonism is relatively uncommon in overdoses but is seen in 6%-18% of toxic ingestions [42]. Our first patient responded to norepinephrine and the second patient did not respond to epinephrine, -in line with previous findings. In this case, however, epinephrine was used as part of the CPR algorithm and not because of hypotension per se. There is no high-quality evidence for preference of catecholamines in the literature. Epinephrine and norepinephrine have both similar beta-1 receptor activity in the heart and similar potency in alpha-receptors. However, norepinephrine has a low beta-2 receptor activity. Therefore, norepinephrine will increase total peripheral resistance more than epinephrine. This may explain why norepinephrine is preferred, and epinephrine not recommended treatment of hypotension in Q-poisonings.
In our first case, the patient went into status epilepticus which did not respond to diazepam treatment alone. This is in accordance with the case reported by Purg, et al. [33]. Our patient needed treatment with sodium valproate in addition to benzodiazepines in order to control the convulsions.
Epidemiology
Most of the cases in the literature were female patients (65%), self-inflicted and by oral route. Moderate poisonings with XR formulation combined with other substances predominated. The reported mortality was estimated to be as high as 13% of the cases. The frequency of reported complications (62%) is in accordance with the high mortality, but not with the low frequency of reported specific treatment procedures (4%). The epidemiological data collected from NPIC in 2015-2019 indicate an overall 36% increase in Q poisonings, a 20% increase in severe Q poisonings, and an increase of Q related deaths (2016-2017; Supplementary material).
Conclusions
The use and misuse of Q are increasing. Clinicians must be aware of serious adverse effects and drug interactions. Toxicokinetic data on Q and NQ indicate that patients with large Q overdosage may profit from gastric lavage and activated charcoal, even later than two hours after ingestion. Moreover, patients may deteriorate quickly and should be monitored closely after admission.
References
- (2018) Quetiapine tablets, summary of Product Characteristics (SPC).
- (2003) Seroquel-Summary of Product Characteristics (SPC) The Norwegian Pharmaceutical Product Compendium (Felleskatalogen AS): Association of Pharmaceutical Industry in Norway (LMI).
- The Norwegian Prescription Database (NorPD) - N05AH04 quetiapin (2019-2009).
- Debernard KAB, Frost J, Ronald PH (2019) Quetiapine is not a sleeping pill. Tidsskr Nor Laegeforen 139.
- Dong-Wook Kim, K-YW, Eon-Pyo Hong, Eun Kyoung Chung, Kyung-Tae Lee (2016) Comparative Physicochemical and Pharmacokinetic Properties of Quetiapine and its Active Metabolite Norquetiapine. Chem Pharm Bull 64: 1546-1554.
- Lee J, Pilgrim J, Gerostamoulos D, Robinson J, Wong A (2018) Increasing rates of quetiapine overdose, misuse, and mortality in Victoria, Australia. Drug Alcohol Depend 187: 95-99.
- Arslan ED, Demir A, Yilmaz F, Kavalci C, Karakilic E, et al. (2013) Treatment of quetiapine overdose with intravenous lipid emulsion. Keio J Med 62: 53-57.
- Bartos M, Knudsen K (2013) Use of intravenous lipid emulsion in the resuscitation of a patient with cardiovascular collapse after a severe overdose of quetiapine. Clin Toxicol 51: 501-514.
- Brunetti ND, Ieva R, Correale M, Cuculo A, Santoro F, et al. (2016) Inferior ST-Elevation Acute Myocardial Infarction or an Inferior-Lead Brugada-like Electrocardiogram Pattern Associated With the Use of Pregabalin and Quetiapine?. Am J Ther 23: 1057-1059.
- Capuano A, Ruggiero S, Vestini F, Ianniello B, Rafaniello C, et al. (2011) Survival from coma induced by an intentional 36-g overdose of extended-release quetiapine. Drug Chem Toxicol 34: 475-477.
- Chen JA, Unverferth KM, Cheung EH (2018) Delayed-Onset Seizure in a Mild Quetiapine Overdose: Report of a Case and Review of the Literature. Case Rep Psychiatry 7623051.
- Chiang ST, Lan CC (2018) Quetiapine Related Acute Paralytic Ileus in a Bipolar I Disorder Patient with Successful Low Dose Amisulpride Substitution: A Case Report. Clin Psychopharmacol Neurosci 16: 228-231.
- Chiou YJ, Lee Y, Lin CC, Huang TL (2015) A Case Report of Catatonia and Neuroleptic Malignant Syndrome With Multiple Treatment Modalities: Short Communication and Literature Review. Medicine 94: e1752.
- Christodoulou C, Margaritis D, Makris G, Kavatha D, Efstathiou V, et al. (2015) Quetiapine and clarithromycin-induced neuroleptic malignant syndrome. Clin Neuropharmacol 38: 36-37.
- Cole JB, Stellpflug SJ, Ellsworth H, Harris CR (2012) Reversal of quetiapine-induced altered mental status with physostigmine: a case series. Am J Emerg Med 30: 950-953.
- Devresse A, Maldague P, Coulier B, Pierard F, Gielen I (2012) Focal parietal necrosis of the sigmoid due to atypical neuroleptics: A case report. Acta Gastroenterol Belg 75: 263-265.
- Detweiler MB, Sullivan K, Sharma TR, Kim KY, Detweiler JG (2013) Case reports of neuroleptic malignant syndrome in context of quetiapine use. Psychiatr Q 84: 523-541.
- Dickmann JR, Dickmann LM (2010) An uncommonly recognized cause of rhabdomyolysis after quetiapine intoxication. Am J Emerg Med 28: 1060. 1-1060.
- El-Gaaly S, St John P, Dunsmore S, Bolton JM (2009) Atypical neuroleptic malignant syndrome with quetiapine: a case report and review of the literature. J Clin Psychopharmacol 29: 497-499.
- Eren Cevik S, Tasyurek T, Guneysel O (2014) Intralipid emulsion treatment as an antidote in lipophilic drug intoxications. Am J Emerg Med 32: 1103-1108.
- Eyer F, Pfab R, Felgenhauer N, Strubel T, Saugel B, et al. (2011) Clinical and analytical features of severe suicidal quetiapine overdoses--a retrospective cohort study. Clin Toxicol (Phila) 49: 846-853.
- George M, Haasz M, Coronado A, Salhanick S, Korbel L, et al. (2013) Acute dyskinesia, myoclonus, and akathisa in an adolescent male abusing quetiapine via nasal insufflation: a case study. BMC Pediatr 13: 187.
- Gibiino S, Trappoli A, Balzarro B, Atti AR, De Ronchi D (2015) Coma After Quetiapine Fumarate Intentional Overdose in a 71-year-old Man: A Case Report. Drug Saf Case Rep 2: 3.
- Giuntoli L, Dalmastri V, Cilloni N, Orsi C, Stalteri L, et al. (2019) Severe quetiapine voluntary overdose successfully treated with a new hemoperfusion sorbent. Int J Artif Organs 42: 516-520.
- Hantson P, Di Fazio V, Wallemacq P (2010) Toxicokinetic interaction between quetiapine and antiretroviral therapy following quetiapine overdose. Drug Metab Lett 4: 7-8.
- Lannemyr L, Knudsen K (2012) Severe overdose of quetiapine treated successfully with extracorporeal life support. Clin Toxicol (Phila) 50: 258-261.
- Liolios A, Sentissi O (2012) Rhabdomyolysis following Acute Extended-Release Quetiapine Poisoning: A Case Report. Case Rep Psychiatry 2012: 347421.
- Nincevic Z, Lasic D, Glavina T, Mikacic M, Carev M, et al. (2017) Quetiapine Poisoning Associated with Neuroleptic Malignant Syndrome, Rhabdomyolysis and Renal Failure: A Case Report. Psychiatr Danub 29: 84-86.
- Papazisis G, Mastrogianni O, Chatzinikolaou F, Vasiliadis N, Raikos N (2012) Sudden cardiac death due to quetiapine overdose. Psychiatry Clin Neurosci 66: 535.
- Paulzen M, Grunder G, Orlikowsky T, Graf CM, Hoeltzenbein M, et al. (2015) Suicide attempt during late pregnancy with quetiapine: nonfatal outcome despite severe intoxication. J Clin Psychopharmacol 35: 343-344.
- Peridy E, Hamel JF, Rolland AL, Gohier B, Boels D (2019) Quetiapine Poisoning and Factors Influencing Severity. J Clin Psychopharmacol 39: 312-317.
- Pilgrim JL, Drummer OH (2013) The toxicology and comorbidities of fatal cases involving quetiapine. Forensic Sci Med Pathol 9: 170-176.
- Purg D, Markota A, Grenc D, Sinkovic A (2016) Low-dose intravenous lipid emulsion for the treatment of severe quetiapine and citalopram poisoning. Arh Hig Rada Toksikol 67: 164-166.
- Rauber-Luthy C, Hofer KE, Bodmer M, Kullak-Ublick GA, Kupferschmidt H, et al. (2013) Gastric pharmacobezoars in quetiapine extended-release overdose: a case series. Clin Toxicol (Phila). 51: 937-940.
- Rhyee SH, Pedapati EV, Thompson J (2010) Prolonged delirium after quetiapine overdose. Pediatr Emerg Care 26: 754-756.
- Skov L, Johansen SS, Linnet K (2015) Postmortem Quetiapine Reference Concentrations in Brain and Blood. J Anal Toxicol 39: 557-561.
- Teo DCL, Wong HK, Tan SN (2018) Atypical Neuroleptic Malignant Syndrome Precipitated by Clozapine and Quetiapine Overdose: A Diagnostic Challenge. Innov Clin Neurosci 15: 20-22.
- Uncles DR, Willers J, Sable N, Finn SD (2010) Gift of the glob goes global. Anaesthesia 65: 209-210.
- Nielsen AS, Damek DM (2012) Window of opportunity: flexion myelopathy after drug overdose. J Emerg Med 42: 36-39.
- Fond G, MacGregor A, Ducasse D, Brittner M (2014) Paradoxical severe agitation induced by add-on high-doses quetiapine in schizo-affective disorder. Psychiatry Res 216: 286-287.
- Taylor L, Graudins A (2019) Extended-release quetiapine overdose is associated with delayed onset of toxicity compared to immediate-release quetiapine overdose. Emerg Med Australas 31: 562-568.
- Fisher J, Taori G, Braitberg G, Graudins A (2014) Methylene blue used in the treatment of refractory shock resulting from drug poisoning. Clin Toxicol (Phila) 52: 63-65.
- Garg V, Farah N (2010) Quetiapine overdose. Aust N Z J Psychiatry 44: 1144.
- Hughes RL (2019) Fatal combination of mitragynine and quetiapine - a case report with discussion of a potential herb-drug interaction. Forensic Sci Med Pathol 15: 110-113.
- Khalid M, Bakhit A, Dufresne A, Sapkota D, Altekreti A (2017) LBBB Induced by Quetiapine Overdose: A Case Report and Literature Review. Am J Ther 24: 618-620.
- Müller C RH, Dohmen C (2009) Intoxication after extreme oral overdose of quetiapine to attempt suicide: pharmacological concerns of side effects. Case Rep Med 2009: 371698.
- (2005) Administration FaD. FDA - Seroquel. In: Administration FaD, editor.
- Seroquel depot - Summary of Product Characteristic (SPC) The Norwegian Pharmaceutical Product Compendium (Felleskatalogen AS): Association of Pharmaceutical Industry in Norway (LMI).
- Figueroa C BM, Hamer-Maansson JE, Winter H (2009) Pharmacokinetic profiles of extended release quetiapine fumarate compared with quetiapine immediate release. Progress in neuro psychopharmacology & biological psychiatry 33: 199-204.
- Pollak PT, Zbuk K (2000) Quetiapine fumarate overdose: clinical and pharmacokinetic lessons from extreme conditions. Clin Pharmacol Ther 68: 92-97.
- A Ngo, Ciranni MC, Olson KR (2008) Acute quetiapine overdose in adults: A 5-year retrospective case series. Ann Emerg Med 52: 5 41-547.
- Hunfeld NG WE, Boswijk DJ, de Haas JA, van Putten MJ, Touw DJ (2006) Quetiapine in overdosage: A clinical and pharmacokinetic analysis of 14 cases. Ther Drug Monit 28: 185-189.
- Trenton A CG, Zwemer F (2003) Fatalities associated with therapeutic use and overdose of atypical antipsychotics. CNS Drugs 17: 307-324.
- Fernandes PP, William M (2002) Death associated with quetiapine overdose. Am J Psychiatry 159: 2114.
- Isbister GK, Duffull S (2009) Quetiapine overdose: Predicting intubation, duration of ventilation, cardiac monitoring and the effect of activated charcoal. Int Clin Psychopharmacol 24: 174-180.
- Hawkins DJ, Unwin P (2008) Paradoxical and severe hypotention in response to adrenaline infusions in massive quetiapine overdose. Crit Care Resusc 10: 320-322.
- (2019) Centre NPI. Quetiapine - treatment recomendation for poisoning. The Norwegian Electronic Health Library Norwegian Poison Information Centre.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...