Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1725
Research Article(ISSN: 2641-1725) 
Extraction and Determination of Anti-Oxidant Activity of Polyphenols from Carrot Pomace, and Their Use in Date Oat Bar Volume 2 - Issue 3
Hammad Naeem1* , Umar Niaz2 and Sarmad Sattar1
- 1National Institute of Food Science and Technology, Pakistan
- 2 Department of Entomology, Pakistan
Received: November 27, 2018; Published: December 03, 2018
*Corresponding author: Hammad Naeem, National Institute of Food Science and Technology, Pakistan
DOI: 10.32474/LOJMS.2018.02.000138
Abstract
- Abstract
- Introduction
- Objectives
- Materials and Methods
- Determination of Moisture Content in Carrot Pomace Powder
- Determination of Ash in Carrot Pomace Powder
- Determination of Crude Fiber Content in Carrot Pomace Powder
- Determination of Fat Content in Carrot Pomace Powder
- Extraction of Polyphenols
- Determination of Total Polyphenol Content
- Determination of Antioxidant Activity of Carrot Pomace Powder
- References
Carrot pomace has polyphenols in large amounts. Polyphenols play an important role in the prevention of degenerative diseases such as cancer and cardiovascular diseases. Carrot pomace goes to waste with all these useful compounds. Carrot pomace or its extract can be utilized in food products to combat food shortage. Extraction of molecules from biological materials by conventional techniques, such as simple maceration, is time consuming. Sonication breaks the cell membranes. It reduces considerably the extraction time and increasing the extract yield. These phenolics were extracted using conventional Maceration techniques & advance Sonication technique. The extracts were used in Functional date-oat bars. Bars were then tested for determination of total phenolics present in them. Total Polyphenol content of functional date bar having 1% extract were 3.160 mg GAE/g of extract. Antioxidant activity in terms of % inhibition of functional date bar having 1% extract is 26.70%. It is evident from the results that the extracts of carrot pomace contain significant number of polyphenols and anti-oxidant activity. The potential of reducing waste to combat the food security issues are high, as it is estimated that by 50% reduction in current food waste, the world would be saving 1314 trillion kcal per year. This represent a reduction of about 22% of the number of additional calories needed to feed the projected population by 2050.
Introduction
- Abstract
- Introduction
- Objectives
- Materials and Methods
- Determination of Moisture Content in Carrot Pomace Powder
- Determination of Ash in Carrot Pomace Powder
- Determination of Crude Fiber Content in Carrot Pomace Powder
- Determination of Fat Content in Carrot Pomace Powder
- Extraction of Polyphenols
- Determination of Total Polyphenol Content
- Determination of Antioxidant Activity of Carrot Pomace Powder
- References
The carrot belongs to the family Apiaceae. The carrot originated in Asia. Carrots are particularly rich in carotene (pro-vitamin A) and also significant amount of polyphenols. Carrots are also known to have polyphenols and antioxidants. Carrots are consumed either fresh, as a salad crop, or cooked. Large quantities are also processed, either alone or in mixtures with other vegetables, by canning or freezing. Polyphenols are a group of chemical substances found in plants. Polyphenols are naturally present in plants. Polyphenols are not essential nutrients meaning that they are not required by the human body for sustaining life, but they can exert beneficial functions [1]. Fruits and vegetables are sources of polyphenols. Polyphenols play an important role in the prevention of degenerative diseases such as cancer and cardiovascular diseases. Polyphenols are antioxidants. Antioxidants are the substances that prevent oxidation. Antioxidants, polyphenols and carotenoids, may help protect cells from damage caused by free radicals. Extraction of molecules from biological materials by conventional techniques, such as simple maceration, is time consuming. The development of modern techniques such as extraction assisted by microwave or extraction assisted by ultrasound. Sonication breaks the cell membranes. It reduces considerably the extraction time and increasing the extract yield. The application of ultrasound disrupts the cell wall structures and accelerates diffusion through membranes; thus, the cell lyses and hence facilitates the release of cell contents. The potential of reducing waste to combat the food security issues are high, as it is estimated that by 50% reduction in current food waste, the world would be saving 1314 trillion kcal per year. This represent a reduction of about 22% of the number of additional calories needed to feed the projected population by 2050. By the year 2100, the global population is expected to increase to 11.2 billion. To feed this projected population and address the food security and the environmental issues, waste reduction and utilization food resources are the important strategies to be developed. The nutritional characterization of waste from fruits and vegetables suggest that most of these crop remains and by products can be utilized, recovered and converted into value added food products. Hence, value addition of these wastes through drying technology and extraction methods to dehydrated products and nutraceutical products, respectively could be an alternative market option for the food and the associated industries. We incorporated phenolic extract in date bars so that we can get benefit from their anti-oxidant properties. Date bars have high nutrition value. Consumer prefer date bars that are more tasted followed by proper textural features that could be obtained by equilibrium of ingredients. Antioxidant activity resulting from the presence of phenolic compounds in the bars is well established.
Objectives
- Abstract
- Introduction
- Objectives
- Materials and Methods
- Determination of Moisture Content in Carrot Pomace Powder
- Determination of Ash in Carrot Pomace Powder
- Determination of Crude Fiber Content in Carrot Pomace Powder
- Determination of Fat Content in Carrot Pomace Powder
- Extraction of Polyphenols
- Determination of Total Polyphenol Content
- Determination of Antioxidant Activity of Carrot Pomace Powder
- References
a) To carry out proximate analysis of carrot pomace powder.
b) To determine the polyphenol content of carrot pomace extract.
c) To determine the Antioxidant activity of carrot pomace extract.
d) To produce functional date bars.
Materials and Methods
- Abstract
- Introduction
- Objectives
- Materials and Methods
- Determination of Moisture Content in Carrot Pomace Powder
- Determination of Ash in Carrot Pomace Powder
- Determination of Crude Fiber Content in Carrot Pomace Powder
- Determination of Fat Content in Carrot Pomace Powder
- Extraction of Polyphenols
- Determination of Total Polyphenol Content
- Determination of Antioxidant Activity of Carrot Pomace Powder
- References
The research study was conducted at Food Sciences Research Institute (FSRI), National Agriculture Research Centre (NARC), Islamabad, Pakistan.
a. Selection of vegetables: Carrot vegetable were purchased from local vegetable market, Islamabad and transferred to FSRI, NARC.
b. Preparation of pomace powder: Vegetables were washed thoroughly, juice was extracted, and fresh weight of carrot pomace was taken on calibrated Top load balance. (Seedburo 8800A).
c. Drying of carrot pomace: Carrot pomace were sun dried for 10 hours and then carrot pomace dried in hot air oven (Mermmet ULM-500) at 5000C for 48 hours until moisture content of carrot pomace was reduced to 10% or below. Dried carrot pomace was weighed on top load balance (Figure 1).
d. Grinding of Carrot Pomace: Dried carrot pomace was grounded to fine powder in a Cyclotech mill with a sieve size 0.5 mm and weighed.
e. Storage of pomace powder: The pomace powder was packed in air-tight zip bags and stored at refrigeration temperature 400C until further studies (Figure 2).
Determination of Moisture Content in Carrot Pomace Powder
- Abstract
- Introduction
- Objectives
- Materials and Methods
- Determination of Moisture Content in Carrot Pomace Powder
- Determination of Ash in Carrot Pomace Powder
- Determination of Crude Fiber Content in Carrot Pomace Powder
- Determination of Fat Content in Carrot Pomace Powder
- Extraction of Polyphenols
- Determination of Total Polyphenol Content
- Determination of Antioxidant Activity of Carrot Pomace Powder
- References
Sample is weighed and placed in hot air oven with set temperature at 1300C for 1 hour (60 minutes). The weight after drying is used to determine moisture content of the sample (Figure 3).
Apparatus:
i. Hot air oven (Memmert OVEN FML-13)
ii. Moisture dishes
iii. Desiccator
iv. Plastic spatula
v. Analytical weighing balance
Procedure:
Took two moisture dishes and labelled them. Take 2g of carrot pomace powder in these moisture dishes. Note the weight of empty moisture dishes and exact weight of sample taken. Now cover the moisture dishes with lids and transferred them to hot air oven and now uncovered them [2,3]. Set the temperature at 1300C and note the time when temperature reached at 1300C. After 1 hour (60 minutes) turned off the oven and put the moisture dishes out from the oven with the help of gloves and covered the dishes with lids. Now place the moisture dishes in desiccator for 15-25minutes (Figures 4-6). After, moisture dishes are cooled to room temperature. Remove the lids and weighed them again (Tables 1 & 2).
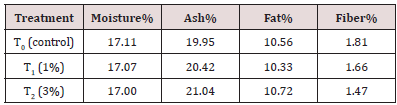
Table 1: Proximate analysis of carrot pomace powder. It is evident from table 2 that Carrot pomace powder has optimum composition as described by other researchers.
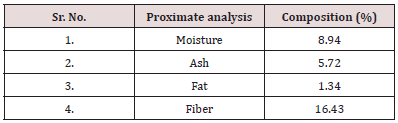
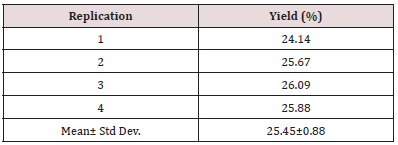
Table 2: Yield of carrot pomace extract. The yield of carrot pomace extract is within range as described by earlier studies.
Calculation:
%moisture= InitialWeight FinalWeight/ sampleweight * 100
Determination of Ash in Carrot Pomace Powder
- Abstract
- Introduction
- Objectives
- Materials and Methods
- Determination of Moisture Content in Carrot Pomace Powder
- Determination of Ash in Carrot Pomace Powder
- Determination of Crude Fiber Content in Carrot Pomace Powder
- Determination of Fat Content in Carrot Pomace Powder
- Extraction of Polyphenols
- Determination of Total Polyphenol Content
- Determination of Antioxidant Activity of Carrot Pomace Powder
- References
Principle:
At high temperature i.e. 5500C all organic compounds are burnt off. Minerals are less volatile than other food compounds and are not destroyed at this high temperature. The inorganic material left behind is called ash (minerals).
Apparatus:
a. Muffled furnace (CARBOLITE AAF 1100)
b. Crucibles with lids
c. Desiccator
d. Analytical weighing balance
Procedure:
Took clean crucibles at room temperature and weighed them without their lids. Now take 3g of carrot pomace powder. Note the weight accurately. Now place the lids on the crucibles and transferred them to the Muffled furnace [4]. Set the temperature at 5500C and let the sample incinerate for about 18 hours (overnight) until light whitish grey ash was obtained (Figure 7). Carefully remove the crucibles with the help of gloves and place them in Desiccator for 15-25 minutes. When the crucibles have reached room temperature weighed them accurately (Tables 3,4).
Table 4: Total polyphenols content of carrot pomace extract (Maceration). It is evident from table 4 & 5 that Extraction of total Polyphenol content obtained from Maceration technique was 6.11% higher than Ultrasound assisted extraction. Carrot pomace extract had high enough polyphenols content suitable for further study and preparation of functional foods.
Calculation:
%Ash= FinalWeight CrucibleWeight/ sampleweight * 100
Determination of Crude Fiber Content in Carrot Pomace Powder
- Abstract
- Introduction
- Objectives
- Materials and Methods
- Determination of Moisture Content in Carrot Pomace Powder
- Determination of Ash in Carrot Pomace Powder
- Determination of Crude Fiber Content in Carrot Pomace Powder
- Determination of Fat Content in Carrot Pomace Powder
- Extraction of Polyphenols
- Determination of Total Polyphenol Content
- Determination of Antioxidant Activity of Carrot Pomace Powder
- References
Principle:
Crude fiber is insoluble and combustible organic residue that remains after the sample has been treated under known conditions i.e. 0.25N Sulphuric acid and 40.7% NaOH solution.
Materials and Apparatus:
A. Sulphuric acid 0.25 N
B. Sodium hydroxide 407 g/L (40.7%)
C. Distilled water
D. Crude fiber Digestion apparatus
E. Muslin cloth
F. Oven
G. Muffled furnace
H. Measuring cylinders
I. Beakers
Procedure:
Weighed an empty crucible. Weighed 2g of sample in crucible. Put the sample in beaker. Added 200mL Sulphuric acid. Boiled for 30 minutes. Turned off the heat and add 10ml of sodium hydroxide. Boiled for another 30 minutes. Removed and filtered through muslin cloth(Tables 5 & 6). Washed the residue with hot distilled water to remove excess of alkali. Dried the crucible with residue at 130oC for 1 hour [5]. Let it cool and weighed (A). Ignited the residues at 600oC in muffled furnace overnight (Figure 8).
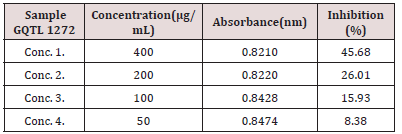
Table 6: DPPH Scavenging activity of polyphenols of carrot pomace extract (maceration). It is evident from table 6 & 7 that as the concentration of the Extract is decreased, the inhibition also decreases with slowed the decrease in antioxidant activity of carrot pomace extract.
Weight of crucible + Sample after drying (A)
Weight of crucible + Sample after ashing (B)
Calculations:
%Crudefiber= A-B/sampleweight * 100
Determination of Fat Content in Carrot Pomace Powder
- Abstract
- Introduction
- Objectives
- Materials and Methods
- Determination of Moisture Content in Carrot Pomace Powder
- Determination of Ash in Carrot Pomace Powder
- Determination of Crude Fiber Content in Carrot Pomace Powder
- Determination of Fat Content in Carrot Pomace Powder
- Extraction of Polyphenols
- Determination of Total Polyphenol Content
- Determination of Antioxidant Activity of Carrot Pomace Powder
- References
Principle:
The fat (triglycerides) can be determined by extracting the samples with suitable solvent e.g. hexane, petroleum ether etc. in a continuous extraction apparatus. Solvent is recollected, and remaining fat is oven dried and weighed.
Material and Apparatus:
a) Analytical balance
b) Buchi apparatus system (B-740)
c) Beakers
d) Cellulose Thimble
e) Desiccator
f) Oven
g) n hexane
Procedure:
3g of sample was weighed in to thimble and covered with tissue paper. Buchi classical beakers were dried at 105oC for 30minutes. Cooled and weighed (W1). 35-40mL of solvent was added. Thimble and beaker were fixed in apparatus. Boiling position was set for 45 minutes. Rinsing position was set for 35minutes (Figure 9). The solvent was collected by blocking the extraction outlet. Beaker with fat was dried at 105oC for 30minutes and cooled in desiccator and weighed (W2) (Tables 7 & 8).
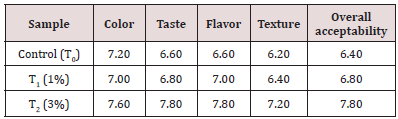
Table 8: Sensory Evaluation of functional date bar. It is clear from the table 13 that treatment T2 (3% extract supplementation) was preferred over others and got the highest sensory score while control date bars were least liked and obtained the lowest sensory score.
Calculation:
%Fat= W1-W2/ sampleweight * 100
Extraction of Polyphenols
- Abstract
- Introduction
- Objectives
- Materials and Methods
- Determination of Moisture Content in Carrot Pomace Powder
- Determination of Ash in Carrot Pomace Powder
- Determination of Crude Fiber Content in Carrot Pomace Powder
- Determination of Fat Content in Carrot Pomace Powder
- Extraction of Polyphenols
- Determination of Total Polyphenol Content
- Determination of Antioxidant Activity of Carrot Pomace Powder
- References
Ultrasound-Assisted Extraction
Principle:
Sonicator produces ultrasonic waves which break the sample at molecular level. The solvent penetrates the sample and polyphenols are dissolved in the solvent.
Apparatus:
i. Sonicator
ii. Beakers
iii. Reagent bottles
iv. Glass funnels
v. Whatman filter paper 41
vi. Aluminum foils
vii. Round Bottom flasks
viii. Volumetric cylinder
Chemicals:
a) Ethanol (50% solution)
b) Methanol (50% solution)
Procedure:
Carefully weighed 3g carrot pomace powder and put it in each of the 4 reagent bottles (125mL). In 2 reagent bottles put 50% ethanol solution and in 2 reagent bottles was added 50% methanol solution. Sample to solvent ratio must be 1:20 [6]. Reagent bottles were closed tightly with their lids (Figure 10). Distill water was added in sonicator up to optimum level. Reagent bottles were carefully placed in sonicator. Sonicator was turned on for 1 hour at 50oC. After 1-hour reagent bottles were taken out from sonicator and cooled to room temperature (Tables 9 & 10).
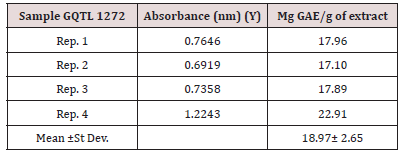
Table 9: Determination of Total Polyphenol Content of functional date bars. It is evident from table no. 9 that polyphenol content of functional date bar having 3% extract is more than the functional date bar having 1% extract.
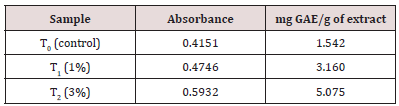
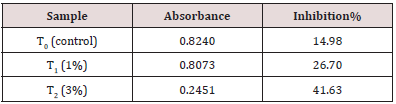
Table 10: Determination of Antioxidant activity of functional date bar. It is clear from table no. 10 that anti-oxidant activity of functional date bar having 3% extract is almost 2 times higher than the functional date bar having 1% extract.
Filtration:
Procedure:
4 round bottom flasks were placed on their respective supports. Samples from sonicator were filtered into round bottom flasks using whatman filter paper no.41.
Solvent extraction:
Apparatus:
A. Rotary evaporator (BUCHI B-490)
B. Circulating chillier
C. Vacuum controller
D. Hot air oven
E. Beakers
F. Aluminum foil
Procedure:
Distilled water was added in water bath to optimum level. Water bath temperature was set at 50oC. Chiller was turned on and its temperature was set at 20oC. Pressure of vacuum controller was set at 120mbar. Round bottom flask was attached with condenser and rotator. Rotation was turned on. Solvent was evaporated and condensed in the condenser and collected in collection chamber. Solvent was evaporated until 10mL of sample remained. This procedure was repeated for each round bottom flask containing sample. Remaining extract of carrot pomace powder was collected in 30mL beakers (Figure 11). These beakers were placed in hot air oven at 45oC for 16 hours to completely evaporate remaining water. Each of the beaker was covered with aluminum foil and placed in refrigerator till further analysis.
Maceration Extraction Technique:
Principle:
Solution containing sample is placed in shaking water bath for 22 hours at 40oC. Shaking water bath agitates the sample thus, enhancing the extraction of polyphenols.
Apparatus and Chemicals:
a. Shaking water bath
b. Ethanol (50% solution)
c. Methanol (50% solution)
d. Carrot pomace powder
e. Analytical Balance (Model 8800A)
f. Conical Flasks
g. Aluminum foil
Procedure:
Procedure:
Determination of Total Polyphenol Content
- Abstract
- Introduction
- Objectives
- Materials and Methods
- Determination of Moisture Content in Carrot Pomace Powder
- Determination of Ash in Carrot Pomace Powder
- Determination of Crude Fiber Content in Carrot Pomace Powder
- Determination of Fat Content in Carrot Pomace Powder
- Extraction of Polyphenols
- Determination of Total Polyphenol Content
- Determination of Antioxidant Activity of Carrot Pomace Powder
- References
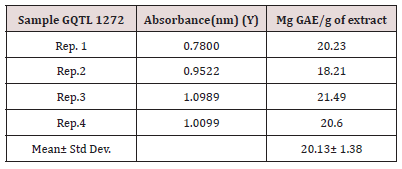
The total polyphenol content of carrot pomace powder was determined by Folin-Ciocalteu method as explained by Singleton (1999). Gallic acid standard solutions were prepared at different concentrations (12.5, 25, 50, 100, 200, 400 μg/mL). Ethanolic solution of sample extract 10mg/mL was prepared for the analysis. 0.5mL ethanolic solution was mixed with 2.5mL of 10% folinciocalteu’s reagent dissolved in water and 2.5mL 7.5% Na2Co3. Blank was simultaneously prepared, containing 0.5mL ethanol, 2.5mL diluted Folin-ciocalteu reagent dissolved in water and 2.5mL 7.5% Na2Co3. Then the sample was incubated at 25oC for 30 minutes. The calibration curve was prepared from various concentrations of Gallic acid standard solutions (Figure 13). The absorbance was determined at 765nm with UV-visible Spectrophotometer. The total polyphenol contents were expressed as mg Gallic acid equivalent (GAE)/g of extract.
Determination of Antioxidant Activity of Carrot Pomace Powder
- Abstract
- Introduction
- Objectives
- Materials and Methods
- Determination of Moisture Content in Carrot Pomace Powder
- Determination of Ash in Carrot Pomace Powder
- Determination of Crude Fiber Content in Carrot Pomace Powder
- Determination of Fat Content in Carrot Pomace Powder
- Extraction of Polyphenols
- Determination of Total Polyphenol Content
- Determination of Antioxidant Activity of Carrot Pomace Powder
- References
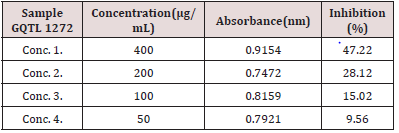
Anti-oxidant activity of carrot pomace extract was determined by DPPH (1, 1-diphenyl-2-picryl-hydrazyl) radical scavenging assay according to method of Brand William et al. (1995) with some modifications. The DPPH Stock Solution was prepared by dissolving 0.006g of DPPH with 25mL of methanol. Carrot pomace extract of concentrations 400μg/ml, 200μg/ml, 100μg/ml, 50μg/ ml were prepared [8,9]. The working solution was obtained DPPH solution with methanol to obtain an absorbance of 0.9921 at 517nm using UV-visible spectrophotometer (Figure 14). 3mL from above mentioned working DPPH solution was mixed with 200μL of the samples at different concentrations (50-400μg/mL). The solution in the test tubes were shaken well and incubated in dark for 15 minutes at room temperature. Absorbance for all the test tubes was taken at 517nm. The Scavenging activity was estimated based on the percentage of DPPH radical.
Preparation of Functional Date Bars
Ingredients:
A. For Control:
i. Dates: 60%
ii. Oats: 20%
iii. Almonds: 16%
iv. Coconut oil: 4%
B. For T1 (1%):
i. Dates: 59%
ii. Oats: 20%
iii. Almonds: 16%
iv. Coconut oil: 4%
v. Extract: 1%
C. For T2 (3%):
i. Dates: 57%
ii. Oats: 20%
iii. Almonds: 16%
iv. Coconut oil: 4%
v. Extract: 3%
Preparation:
Almonds and oats were ground well in a grinder. Carrot extract were added at different ratio (1%, 3%). Dates were added into the grinder. Coconut oil was added into the grinder. All the ingredients were ground well. Transferred all the mixture into tray and placed the tray into refrigerator for 10 minutes. Removed tray out from refrigerator and cut the mixture into bars of 7.5cm length, 2.5cm width, 1cm height. Bars were packed in butter paper.
Sensory Evaluation of Functional Date Bars:
Sensory evaluation of these functional date bars was done for color, taste, flavor, texture and overall acceptability by a panel consisting of 5 judges male and female of different age groups and background. Sample were presented in successions and panelist were asked to rate evaluation variables according to 9-point. Hedonic scale (1=dislike extremely and 9= like extremely) (Figures 15-19).
Proximate Analysis of Functional Date Bars:
The proximate analysis of functional date bars include moisture, ash, fat, crude fiber was carried out according to standard methods of AOAC (2010).
Determination of Total Polyphenol Content:
Polyphenol content of functional date bars were determined by the same method as determined from carrot pomace powder which has been described above.
Extraction of Polyphenols from Functional Date Bars:
Procedure for extraction of polyphenols from functional date bars is same as extraction of polyphenols in carrot pomace powder which has been described above.
Determination of Antioxidant Activity of Functional Date Bars:
Procedure for determination of antioxidant activity from functional date bars is same as determination of antioxidant activity from carrot pomace powder which has been described above.
References
- Abstract
- Introduction
- Objectives
- Materials and Methods
- Determination of Moisture Content in Carrot Pomace Powder
- Determination of Ash in Carrot Pomace Powder
- Determination of Crude Fiber Content in Carrot Pomace Powder
- Determination of Fat Content in Carrot Pomace Powder
- Extraction of Polyphenols
- Determination of Total Polyphenol Content
- Determination of Antioxidant Activity of Carrot Pomace Powder
- References
- https://www.sciencedirect.com/topics/agricultural-and-biologicalsciences/ polyphenol
- MT Lee, WC Lin,B Yu, TT Lee (2017) Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals - A review. Asian-Australas J Anim Sci 30(3): 299-308.
- https://www.researchgate.net/profile/Arvind_Singh56/post/ Does_animal_matter_decompose_faster_than_the_plant_matter/ attachment/59d62ada79197b80779893df/AS:340839160139780@14 58273796218/download/Decomposition.pdf
- https://www.teaclass.com/lesson_0301.html
- Daniel Hinojosa Nogueira, Joaquín Muros, José A Rufián Henares, Silvia Pastoriza (2017) New Method to Estimate Total Polyphenol Excretion: Comparison of Fast Blue BB versus Folin-Ciocalteu Performance in Urine. J Agric Food Chem 65(20): 4216-4222.
- http://frenchscout.com/polyphenols
- http://shodhganga.inflibnet.ac.in/bitstream/10603/37756/3/012_ chapter%202.pdf
- Gironi F, Piedmont V (2011) Temperature and solvent effects on polyphenol extraction process from chestnut tree wood. Chemical Engineering Research & Design 89(7A): 857-862.
- http://tolweb.org/treehouses/?treehouse_id=4615

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...