
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2641-1652
Case Report(ISSN: 2641-1652) 
A Chinese Drinking Men Manifested as Liver Cirrhosis with Iron Overload and a History of Leukocytosis Volume 2 - Issue 2
Lizhou Lin*, Rong Wu, Lianfang Du
- Department of Ultrasound, Shanghai General Hospital, Shanghai Jiaotong University School of Medicine, China
Received:April 16, 2019; Published: April 23, 2019
*Corresponding author:Lizhou Lin, Department of Ultrasound, Shanghai General Hospital, China
DOI: 10.32474/CTGH.2019.02.000135
Case History
A 45-year-old man was referral to our hospital for new onset of jaundice and ascites. Symptoms of dark urine, yellow sclera with fatigue and tenderness in the epigastrium started two weeks prior to his admission to local hospital for intensifying yellow sclera and skin with low-grade fever two weeks ago. Liver tests revealed aspartate aminotransferase [AST] 89 [8-40U/L], alanine aminotransferase [ALT] 22 [5-40U/L], total bilirubin [TBIL] 413μmol/L [3.4-11.7μmol/L], direct bilirubin [DBIL] 211.7 [0.0-6.0μmol/L], albumin 25.6 [3.4-5.4g/dL]. Hematological findings revealed white blood cells [WBC] count of 23,950 [4,000-10,000cells/μl], the percent of neutrophile granulocyte [NEUT%] 76.3 [50-70%]. Abdominal MRI showed cirrhosis, ascites and no spleen. He had no serological evidence of hepatitis A-E and was a heavy drunker, thus he was diagnosed as alcoholic cirrhosis and treated previously with hepatic protection drugs and cholagogue drugs. However, the patients’ symptoms had no improvement. The patient had a long history of hepatomegaly when he was 16without any symptom, and he had no treatment. In 2000, due to a trauma, he had a splenectomy. And in 2012, he had a physical examination and found that an obvious increased of WBC [20,000-50,000cells/μl], then he was admitted to do a marrow puncture, considered as infectious bone marrow, treated with anti-infective drugs. The patient had no hypertension, coronary disease, diabetes and variceal bleeding. He had no history of blood transfusion, and no history of drug or food allergy. He was a heavy drunker (1kg white wine per day for more than 20 years), and his family history revealed nothing of note. On physical examination, he had a bronzed face. Cardiac examination revealed a regular rhythm with no extra cardiac sound and cardiac murmurs. Abdominal examination revealed a palpable liver with an edge 2cm below the right costal margin, a 10-cm old surgery scar at the left upper abdomen and no prominent collateral vessels.
Abdominal percussion had a sign of ascites, characterized by a shifting dullness, yet there was no muscular defense or rebound tenderness. Skin examination was notable for dermal and sclera icterus with no liver palms, spider angioma, subcutaneous hemorrhage and lower limbs edema. His mental function was intact, and he had no asterixis. On admission, his liver chemistries were abnormal: AST 76U/L, ALT 34U/L, TBIL 305.5μmol/L, DBIL 159.8μmol/L, AKP 185U/L, GGT 71.0U/L, prothrombin time [PT] 19.7 [9-13sec], international standard time [INR] 1.6 [0.8-1.5], activated partial thromboplastin time [APTT] 49.8 [20-40sec]. His hematological findings were also abnormal: WBC 41,590 cells/μl, NEUT% 70.6%, C-reactive protein 23.0 [0-10mg/dl]. hemoglobin [HGB]11.5g/dl, RBC 297*104 cells/μL, mean corpuscular volume [MCV] 100.7 [83.9-99.1FL], mean corpuscular hemoglobin [MCH] 38.7 [27.8-33.8PG], mean corpuscular hemoglobin concentration [MCHC] 385 [320-360g/L], reticulocyte percentage 3.6 [0.5- 1.5%]. Serum ferritin [SF] 2330.00 [16.0-300.0μg/L], transferrin saturation [TS] 76.3% [33-55%], Vitamin B12 851 [187-883pg/ ml], folic acid 1.3 [3.1-20.5ng/ml], ceruloplasimin 0.243 [0.15- 0.6mg/L]. Peripheral blood smear showed almost normal WBC classification, but RBC morphological character were abnormal with more big mature RBC and 9% target RBC. The values of free hemoglobin concentration and hemoglobin electrophoresis found no abnormality. Bone marrow smear showed that there were granulocytic hyperplasia with little toxicity, erythroid hyperplasia, and macro phagocytic hyperplasia with more platelets, and the neutrophil alkaline phosphatase score was 100. BCR/ABL fusion gene and JAK2/V617F mutation was negative. Bone marrow iron stain: extracellular iron (3+), intracellular iron 92%. Sex hormone test: testosterone 133.55 [270-1570ng/L for adult male]. showed no peripheral nerve injury. M protein electrophoresis was normal. At last, Magnetic Resonance Cholangiopancreatography (MRCP) performed on admission revealed a liver cirrhosis, portal hypertension, ascites, post splenectomy and a significant diffuse decrease of the signal in the liver, heart and pancreas. (Figure 1 & 2), which indicated hemochromatosis.
Figure 1: Magnetic Resonance Cholangiopancreatography (MRCP) findings on admission (as showed red arrows) revealed a liver cirrhosis, portal hypertension, ascites, post splenectomy and a significant diffuse decrease of the signal in the liver and pancreas.
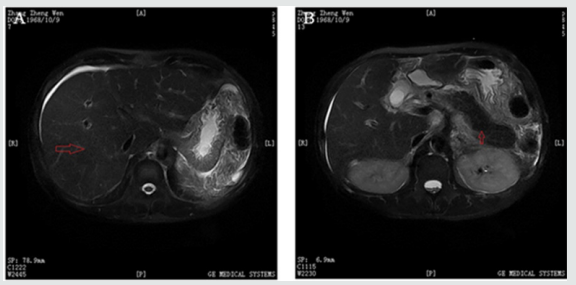
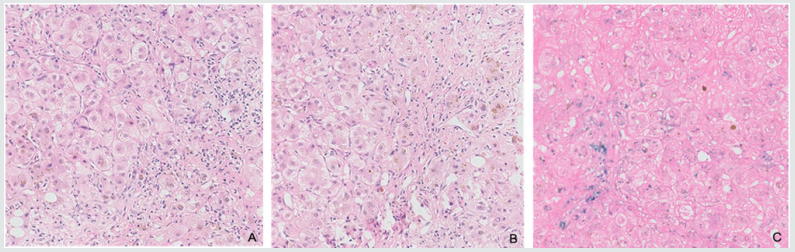
Figure 2: Hematoxylin and eosin image shows pseudolobule formation and normal lobular destroyed with brown particles seen in cytoplasma of hepatocytes. Scattered lymphocytes and plasmocytes were presented in fibrous interstitial without obvious pigmentation; C. Iron stain images shows strong positive reaction in cytoplasma of hepatocytes.
Pathological Findings
A liver biopsy was done after coagulation function improvement. It showed pseudolobule formation and normal lobular destroyed with brown particles seen in cytoplasma of hepatocytes and bile plugs seen in some of bile canaliculi. Scattered lymphocytes and plasmocytes were presented in fiberous interstitial without obvious pigmentation. Iron stain showed strong positive reaction in cytoplasma of hepatocytes and teticular fiber stain showed obvious hyperplasia (Figure 2).
Differential Diagnosis
Since there was no evidence of viral hepatitis, which was the most common cause of cirrhosis, and a solid evidence of high alcohol consumption, the initial diagnosis was most likely to be decompensated alcoholic cirrhosis combined with postsplenectomy leukocytosis, chronic myelogenous leukemia [CML] or spontaneous peritonitis [SBP]. A repeated bone marrow puncture and a peripheral blood smear were done to classify the possibility of more than one-year history of leukocytosis, which exclude the possible diagnosis of CML in view of no primitive cells in blood and bone marrow and negative BCR/ABL fusion gene and JAK2 mutation. No signs of peritoneal irritation and no fall in WBC after antibacterial therapy did not support SBP. The POEMS syndrome [refer to polyneuropathy, prganomegaly, endocrinopathy, M protein, and skin changes] was a consideration in view of skin pigmentation, ascites, hepatomegaly, decreased androgen and thrombocytosis [1]. However, M protein electrophoresis showed no abnormal band and electromyography showed no peripheral nerve injury, which were the two major criteria of POEMS. Other possible causes of cirrhosis should be considered. As mentioned earlier, the patient had no history of medication use ahead of onset, no evidence of extrahepatic cholestasis and blocked flow of hepatic vein. In order to exclude liver cirrhosis caused by metabolic disturbance, SF, TS and ceruloplasimin was tested. We were surprised to find that SF was far more than 1000μg/L and TS was more than 45%, and MRCP revealed iron deposit in liver, heart and pancreases, which indicated hemochromatosis. Liver biopsy showed a characteristic patern with iron acccumulation predominently in hepatocytes, which confirmed the diagnosis of hemochromatosis [2,3]. Secondary hemochromatosis such as haematological disorders (the thalassaemia, sideroblastic anaemias etc.), chronic liver disease (Hepatitis C Virus infection, alcoholic liver disease, nonalcoholic steatohepatitis etc.) should also be in consideration. The thalassaemia was a possibility in view of mild anaemia with 9% of target RBC in peripheral blood and hepatomegaly. However, blood cell analysis did not show microcytic hypochromic anemia and the values of free hemoglobin concentration and hemoglobin electrophoresis found no abnormality. Sideroblastic anaemias was also exclued since bone marrow showed no dyshaematopoesis and ring siderblasts. It was still confusing the reason why WBC had been increased for more than one year. After abstinence and chemotherapy for liver protection, jaundice elimination, iron chelation, WBC declined gradually to almost normal, which pointed that hematopoietic dysfunction might be caused by iron overload and liver cirrhosis. So, it was possible that the patient did have combined pathogenesis of hemochromatosis and alcoholic liver disease advanced the progression to cirrhosis.
Discussion
Hereditary hemochromatosis [HH] is an autosomal recessive, inherited disorder of iron metabolism, which results from mutations in several genes: HFE, TfR2, HJV, FPN and HAMP [4]. The most important hormone of iron homeostasis, hepcidin is regulated by these genes [5], downregulation of which can increase the iron deposition in our body leading to organ damage gradually, especially the liver, the heart and the pancreas, which develops liver fibrosis, cirrhosis, hepatocellular carcinoma, diabetes mellitus, cardiomyopathy, arthritis, and skin pigmentation. Some other iron overload diseases such anaemias (Thalassemic syndromes [6,7], Sideroblastic anaemias, etc.), chronic liver disease (Alcoholic liver disease [8], non-alcoholic fatty liver disease [9], etc.), massive erythrocyte transfusion, etc. Hemochromatosis affects more men than women. It is particularly common in Caucasians of Western European descent with an incidence of 1 in 220 to 250 [10-12] while it is rare in Asians and Blacks. Evidence showed that certain consumption of alcohol per day was associated with serum iron increase [13]. With increasing and persistent alcohol abuse, iron accumulates in liver, which is the primary organ of iron storage, firstly in hepatocytes and lately also in Kupffer cells and macrophages [14]. Alcohol and its metabolites generate reactive oxygen species (ROS) and lipid peroxidation products, which can also be generated by iron [15]. ROS can damage mitochondria, lysosomes, endoplasmic reticulum, and chromosomal DNA leading to fibrosis and cirrhosis. Interestingly, 20-30% of HH were heavy alcoholic so that early investigator had the view that HH was secondary to alcoholic liver cirrhosis until HFE-related genetic mutation was confirmed in HH.
Homozygous HH subjects who consumed more than 60g alcohol per day had remarkable higher prevalence of severe fibrosis or cirrhosis compared with those who consumed less than 60g alcohol per day [16]. Asare and colleagues [17] investigated that iron overload and its co-factor alcohol had multiplicative synergistical interaction in fibrosis, cirrhosis even hepatocellular carcinoma by more oxidative and nitrosative stress, lipid peroxidation and DNA damage. Thus, iron and alcohol act synergistically in causing liver injury. Persistent neutrophilic leukocytosis when the cause is other than leukemia defines a leukemoid reaction [LR], which should mainly exclude chronic myelogenous leukemia and chronic neutrophilic leukemia [18]. The main causes of LR are severe infections, malignancies, intoxication, drugs and other substance including alcohol. Alcohol can stimulate cells to release excessive amounts of necrosis factor-alpa (TNF-α) [19], which was postulated to be instrumental in the cause of LR. Meanwhile, increased iron content in Kupffer Cells can induce the expression and release of pro-inflammatory cytokine TNF-α [20]. Increased serum levels of interleukin (IL)-18 and IL-1β were also found in alcoholic patient, which stimulate proliferation leukocytes in bone marrow both directly and indirectly induction of granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor, thus may be another postulation of LR [21]. After therapy of abstinence, iron chelation and liver protection, WBC of our patient gradually declined to almost normal, which confirm our postulation. In conclusion, the symptoms of hemochromatosis are nonspecific evidences and typically symptoms in the early stages. There is few hemochromatosis reported in china, and patients diagnosed hemochromatosis at last are often found with other complications. In order to early detect, early diagnosis and early treatment, it’s necessary to strengthen the recognition of hemochromatosis, including the patients and the doctors, which improving the patients living quality and life. Further study is warranted to accumulate a higher number of cases.
References
- Li J, Zhou DB (2013) New advances in the diagnosis and treatment of POEMS syndrome. Br J Haematol 161: 303-135.
- Bacon BR, Adams PC, Kowdley KV, Powell LW (2011) Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 54: 328-343.
- Pietrangelo A (2010) Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology 139: 393-408.
- Siddique A, Kowdley KV (2012) Review article: the iron overload syndromes. Aliment Pharmacol Ther 35: 876-893.
- Franchini M (2006) Hereditary iron overload: update on pathophysiology, diagnosis, and treatment. Am J Hematol 81: 202-209.
- Taher A, Hershko C, Cappellini MD (2009) Iron overload in thalassaemia intermedia: reassessment of iron chelation strategies. Br J Haematol 147: 634-640.
- Taher AT, Musallam KM, Cappellini MD, Weatherall DJ (2011) Optimal management of beta thalassaemia intermedia. Br J Haematol 152: 512-523.
- Kohgo Y, Ohtake T, Ikuta K, Suzuki Y (2005) Iron accumulation in alcoholic liver diseases. Alcohol Clin Exp Res 29: 189S-193S.
- Bugianesi E, Manzini P, D Antico S, Vanni E, Longo F, et al. (2004) Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology 39: 179-187.
- Phatak PD, Bonkovsky HL, Kowdley KV (2008) Hereditary hemochromatosis: time for targeted screening. Ann Intern Med 149: 270-272.
- Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, et al. (2005) Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med 352: 1769-1778.
- (2010) EASL clinical practice guidelines for HFE hemochromatosis. J Hepatol 53: 3-22.
- Ioannou GN, Dominitz JA, Weiss NS, Heagerty PJ (2004) The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia. Gastroenterology 126: 1293-1301.
- Kohgo Y, Ohtake T, Ikuta K, Suzuki Y, Hosoki Y, et al. (2005) Iron accumulation in alcoholic liver diseases. Alcohol Clin Exp Res 29: 189S-193S.
- Corradini E, Pietrangelo A (2012) Iron and steatohepatitis. J Gastroenterol Hepatol 27 Suppl 2: 42-46.
- Fletcher LM, Dixon JL, Purdie DM, Powell LW, Crawford DH (2002) Excess alcohol greatly increases the prevalence of cirrhosis in hereditary hemochromatosis. Gastroenterology 122: 281-289.
- Asare GA, Bronz M, Naidoo V, Kew MC (2008) Synergistic interaction between excess hepatic iron and alcohol ingestion in hepatic mutagenesis. Toxicology 254: 11-18.
- Sakka V, Tsiodras S, Giamarellos Bourboulis EJ, Giamarellou H (2006) An update on the etiology and diagnostic evaluation of a leukemoid reaction. Eur J Intern Med 17: 394-398.
- Juturi JV, Hopkins T, Farhangi M (1998) Severe leukocytosis with neutrophilia (leukemoid reaction) in alcoholic steatohepatitis. Am J Gastroenterol 93: 1013.
- She H, Xiong S, Lin M, Zandi E, Giulivi C, et al. (2002) Iron activates NF-kappaB in Kupffer cells. Am J Physiol Gastrointest Liver Physiol 283: G719-26.
- Arguelles Grande C, Leon F, Matilla J, Dominguez J, Montero J (2002) Steroidal management and serum cytokine profile of a case of alcoholic hepatitis with leukemoid reaction. Scand J Gastroenterol 37: 1111-1113.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...

