Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2638-5910
Research Article(ISSN: 2638-5910) 
Differences in participant classification and intervention effects based on HbA1C and fasting plasma glucose among a community sample of African Americans Volume 1 - Issue 5
Lovoria B Williams1*, Jeannette O Andrews2, Stephen W Looney3, Andrea M. Kriska4 and Janie Heath5
- 1Augusta University, College of Nursing
- 2South Carolina, College of Nursing, United States of America
- 3Augusta University, Department of Population Health Sciences, United States of America
- 4University of Pittsburgh, Department of Epidemiology, United States of America
- 5University of Kentucky, College of Nursing, United States of America
Received: November 16, 2018; Published: November 26, 2018
Corresponding author: Lovoria B Williams, College of Nursing, United States of America
DOI: 10.32474/ADO.2018.01.000122
Abstract
Since 2010, the American Diabetes Association has endorsed both HbA1C (A1C) and fasting plasma glucose (FPG) to determine diabetes risk. Given the discordance between classifications based on FPG and A1C and higher A1C levels among African Americans (AAs) than whites, we sought to examine the prevalence of normoglycemia, prediabetes, and diabetes among a community sample of adult AAs (n=704) screened for enrollment in a randomized Diabetes Prevention Program (DPP). We conducted a retrospective analysis to estimate the degree of discordance between risk categories based on FPG and A1C and examine the risk factors that predicted A1C and FPG levels. To determine differential effects on risk categories defined using FPG and A1C, we examined the effects of the program on the measures at 12 weeks post-intervention among the intervention arm participants.
The tests revealed different prevalence levels at baseline: A1C, 36.2% normoglycemia, 52% prediabetes and 11.8% diabetes; FPG, 81.1%, 16.8%, and 2%, respectively. There was discordance among A1C and FPG and among the risk categories (p<0.001). Both final regression models included age and waist circumference as predictors. For FPG, additional predictors were family history of diabetes and male gender. Post intervention, only those classified in the prediabetes category defined in terms of either test demonstrated intervention effects. Screening test choice results in different sample composition; both A1C and FPG respond to intervention effects among individuals classified in the prediabetes group only. These results have implications for research and clinical practice.
Keywords: HbA1C; Fasting plasma glucose; Community based; Screening; African Americans; Prediabetes
Abbreviations: DPP: Diabetes Prevention Program; FPG: fasting plasma glucose; ADA: American Diabetes Association; ARIC: Atherosclerosis Risk in Communities
Introduction
Although the prediabetes rates among African Americans (AAs) and non-Hispanic whites are similar (39% and 35%, respectively), the type 2 diabetes rate among AAs is almost double that of non-Hispanic Whites [1]. Type 2 diabetes develops over a continuum of glycemic progression and beta cell dysfunction. As early as seven years before the diagnosis of type 2 diabetes is made the damaging microvascular effects of dysglycemia such as nephropathy and retinopathy begin to develop [2,3]. Therefore, it is imperative to halt prediabetes and its progression to type 2 diabetes. Data from the Diabetes Prevention Program (DPP) and other community studies demonstrated compelling evidence that lifestyle interventions that target modifiable risk factors such as obesity and sedentary behavior can delay the onset of incident type 2 diabetes among individuals with prediabetes [4,5]. As efforts to continue to implement the DPP evidenced-based programs into community settings, DPP translation teams must carefully consider the appropriate method to use for participant screening. DPP translation teams primarily use the fasting plasma glucose (FPG) to determine participant eligibility. However, since the endorsement of HbA1C (A1C) by the American Diabetes Association (ADA) as a screening test in 2010, A1C has been added as an additional option [6]. Because there are no recommendations regarding the best glucose test choice, it is important to determine how FPG and A1C perform in identifying prediabetes in high-risk individuals such as overweight/obese AAs.
Evidence from prospective cohort and nationally representative cross-sectional studies indicate that the prevalence of prediabetes varies by choice of glucose test [7-9]. Moreover, higher A1C values among AAs are well documented [10-14], yet the results are mixed regarding whether the higher values are reflective of higher diabetes risk [15-17]. These controversies have led to questions regarding the performance of A1C among AAs for screening [18- 20]. To our knowledge, the impact on participant classification of using both A1C and FPG to screen community-based AAs for a DPP-like intervention has not been reported. Given the convenient use of the A1C test compared to FPG, translation teams are likely to increase the use of A1C as a screening method for community based DPPs.
The Purpose of This Analysis was Threefold to
a. Compare the prevalence of normoglycemia, prediabetes, and diabetes among a sample of AAs who were screened for enrollment in a randomized communitybased DPP Group Lifestyle Balance Program (GLB) known as Fit Body and Soul;
b. Estimate the degree of discordance between risk group classifications based on FPG and A1C; and
c. Examine the risk factors that predicted A1C and FPG levels. In addition, we examined the effects of the GLB on the same measures at 12-week post-intervention among the Fit Body and Soul intervention arm [21,22].
Inclusion Criteria for Fit Body and Soul were: self-described AA, ages 20–64 years, non-diabetic (FPG) <126 mg/dl),a body-mass index (the weight in kilograms divided by the square of the height in meters) ≥25, no medical contraindications to physical activity, no history of gastric weight-loss surgery or weight loss of more than 10 % in the past 3 months for any reason other than childbirth, no physical conditions or medications that might affect glucose metabolism, no behaviors that might interfere with participation, no illnesses that would limit life span, and for females, no current pregnancy or planned pregnancy within the study period. The primary outcome measure was weight reduction by week 12. Secondary outcomes included A1C, FPG, anthropometrics, and
Self-Administered Questionnaires: International Physical Activity Questionnaire LF, SF12, and the Euro-Quality of Life. Data collectors obtained outcomes measures at baseline, 12 weeks post-intervention and 12 months post- baseline measurements. A complete description of full methodology and the results of Fit Body and Soul are reported elsewhere.
Materials and Methods
A retrospective data analysis was conducted from the sample of adult AAs who were screened for inclusion in Fit Body and Soul (n=704). The analysis presented here includes all individuals who were screened for study eligibility, including those who were subsequently excluded due to glucose values that exceeded the Fit Body and Soul inclusion criteria. For post-intervention effects, we analyzed the GLB intervention arm only. The study was approved by the Augusta University Institutional Review Board. All participants were consented.
Glucose level was classified as normal, prediabetes, or diabetes according to the ADA guidelines [6]. For A1C, prediabetes was defined as A1C between 5.7% and 6.4% (39 mmol/mol-46 mmol/ mol); normoglycemia, A1C<5.7% (39 mmol/mol); and diabetes, A1C≥6.5% (48 mmol/mol). For FPG, prediabetes was defined as FPG between 100 and 125 mg/dl; normoglycemia, FPG < 100 mg/dl; and diabetes, FPG>125. Multiple linear regression (MLR) analyses were then performed to assess the predictive ability of type 2 diabetes risk factors on both FPG and A1C. A separate MLR model was developed for each dependent variable (A1C and FPG) using forward selection, backward-elimination, and stepwise variable-selection procedures. Final MLR models were assessed for adherence to assumptions using standard statistical tests and detailed graphical analysis of residuals for each model.
To test for significant improvement of A1C and FPG in the GLB intervention arm participants from baseline to 12- weeks post intervention, the Wilcoxin signed-ranks test was used due to the high degree of skewness in the FPG and A1C data. The prediabetes and normoglycemia groups were compared in terms of improvement in A1C and FPG at 12-weeks post intervention by using the Mann- Whitney-Wilcoxon rank-sum test. Mc Nemar’s was used to test for significant discordance between the classifications based on A1C and FPG, and Cohen’s kappa to measure agreement between them. All statistical tests were two-tailed and were performed at the 0.05 level of significance. SPSS, Version 21 (IBM Corp., 2012) was used for all analyses.
Results
The mean age of participants was 46.7 years (SD =10.8. Most were female (82.5%), married (52.0%), employed fulltime (72.7%) and college-educated (83.2%). The mean body mass index was 35.4 (SD = 7.4) and mean waist circumference was 107.8 cm (SD = 15.7). According to A1C, the average participant was categorized in the prediabetes group, mean A1C 5.85% (40 mmol/mol) [SD=0.57]. According to FPG, the average participant was categorized in the normoglycemia group, mean 91.7 mg/dl (SD =13.6).
Discordance between FPG and A1C
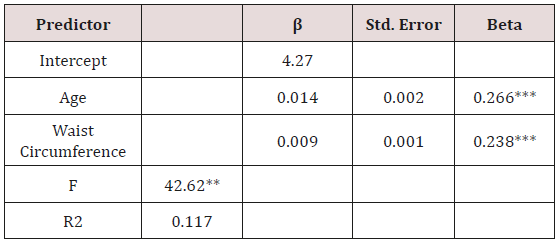
McNemar’s test indicated significant discordance between FPG and A1C in terms of the ADA classifications (p< 0.001). The FPG classified more participants in the normoglycemia category at baseline and the A1C classified more participants in the prediabetes category. The agreement between ADA classifications based on A1C and FPG was weak (Cohen’s Kappa =0.136), as shown in Table 1.
Table 1: Sample Comparison of Participant Classification by Glucose Test (n = 646).
*McNemar Chi Square
Regression Analysis
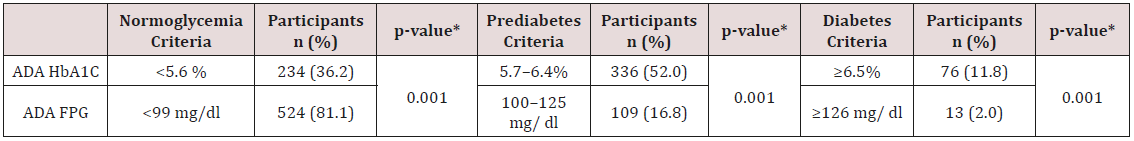
The final MLR model predicting A1C explained 11.7% of the variance and included age and waist circumference as predictors (Table 2). For FPG, the predictors included in the final MLR model were age, waist circumference, family history of diabetes, and male gender, with the model explaining 8.8% of the variance (Table 3).
Table 3: Final Regression Model Predicting Fasting Plasma Glucose (n = 496).
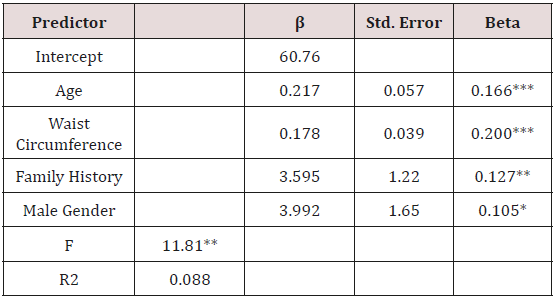
Note: *** p < 0.001; *p < 0.01; *p < 0.05
Analysis of Intervention Effects on A1C and FPG at Week 12 Follow up Among the GLB Arm Participants
Table 4: Change in FPG and A1C from Baseline to 12 Week Follow-Up Among GLB Arm Participants Classified in Prediabetes and Normoglycemia Groups.
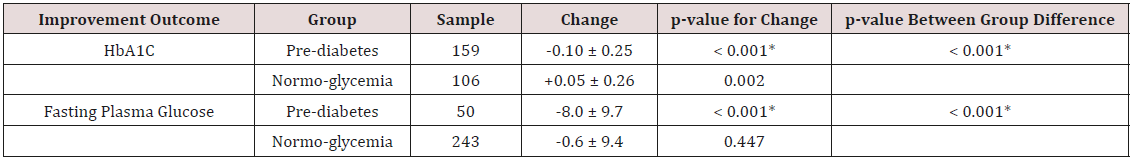
*Significant at p < 0.05.
A1C data were available for 265 (72%) of the 370 participants enrolled in the GLB arm at week 12. Of these participants, 159 (60%) were classified in the prediabetes group and 106 (40%) in the normoglycemia group at baseline. FPG data were available for 293 (79%) of the 370 participants in the GLB arm at week 12. Of these participants, 50 (17%) were classified in the prediabetes group and 243 (83%) in the normoglycemia group at baseline (Table 4). From baseline to week 12, A1C improved significantly (p=< 0.001) among those classified in the prediabetes group, whereas those in the normoglycemia group had a significant increase (p=0.002) in their A1C level. The pre-diabetes group differed significantly from the normoglycemia group in terms of change in A1C from baseline to week 12 (p=0.001). At week 12, the improvement in FPG was significant only among those classified in the prediabetes group. As indicated in Table 4, the FPG improvement in the prediabetes group was significantly greater than the small improvement in the normoglycemia group.
Discussion
We found that screening high-risk AAs with A1C or FPG identified different individuals in the prediabetes category and that there was a significant discordance between the diagnosis of prediabetes based on the A1C and FPG criteria among AAs. These findings are consistent with other reports [9, 23]. Additionally, regardless of the test used, significant glucose improvements occurred among individuals with prediabetes.
Different participant classification according to the screening test used emphasizes the implications of investigator choice of test. According to our analysis, in this sample the A1C identified more individuals in the prediabetes category than FPG. Therefore, the use of FPG may over-classify AAs in the normoglycemia group, which may result in potentially high-risk individuals being excluded from participation in DPPs. On the other hand, the use of A1C may over-identify AA participants as having prediabetes. Higher A1C levels among AAs is consistent with results found in nationally representative samples [14, 24] and in those found in the original DPP study [13]. Although the results are mixed, elevated A1C levels may not be indicative of increased diabetes risk among AAs but may reflect the known higher A1C levels among this population.
Given our regression analysis results and the high prevalence of known diabetes risk factors among AAs such as overweight/obesity, hypertension, and family history of diabetes, community-based DPP translation teams might consider the use of non-invasive diabetes risk assessment tools, such as the ADA or CDC questionnaires to conduct participant screening [25,26]. These simple-to-use pencil and paper tools are easy to apply in the community setting and they have acceptable sensitivity and specificity [27].
Our results are consistent with those of others in that the GLB intervention was most effective among those classified in the prediabetes group [28,29]. To our knowledge, there is no evidence that suggest metabolic benefits of weight loss among AAs with normoglycemia. Moreover, because the majority of individuals with normoglycemia are less likely than those with prediabetes to progress to diabetes, DPP translation teams may consider limiting enrollment to individuals with prediabetes. The clinical significance of elevated A1C levels among AAs remains unclear. Selvin et al. [17] found that elevated A1C was a predictor of renal and cardiovascular outcomes as well as mortality. However, despite the higher baseline A1C values among AAs, there were no differences in these outcomes between AAs and whites.
Lacey et al. [30] included A1C in a 5-year diabetes risk prediction model in a biracial cohort of AA and white adults. Although results indicated that adding the baseline A1C as a predictor to the model improved discrimination in both races, the model discrimination was significantly higher (p=0.008) in whites than AAs. The results of these studies indicate that additional prospective epidemiologic investigations such as the Atherosclerosis Risk in Communities (ARIC) cohort study are warranted to elucidate the predictive value of A1C for incident diabetes and diabetes complications among AAs in community-based samples [31].
Although our study elucidated the impact of choice of screening test on sample composition, it is not without limitations. The current study focus was not the original aim of Fit Body and Soul; therefore, the data were limited by the variables collected during the original study. Additional laboratory tests, such as lipid panel and urine microalbumin might have explained more of the variance in the regression models. Moreover, a gold standard measure such as the oral glucose tolerance test would have provided more information regarding test performance. Despite the limitations, the findings of this study add to the body of literature regarding the impact of DPP translation team’s choice of screening methods when preparing to implement a community-based DPP.
Conclusion
The investigator choice of glucose test results in different sample makeups. However, regardless of the glucose test used, significant glucose improvements occurred only among individuals with prediabetes. These findings support evidence that DPP interventions should be limited to high-risk individuals based on glucose status.
Acknowledgement
The authors have no known conflicts of interest. We acknowledge colleagues Thomas Joshua for his data management support and April Carson for their editorial support.
References
- Centers for Disease Control and Prevention (2017) National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2014. Atlanta, GA: Centers for Disease Control and Prevention.
- Harris MI, Klein R, Welborn TA, Knuiman MW (1992) Onset of NIDDM occurs at least 4-7 yr. before clinical diagnosis. Diabetes Care 15(7): 815- 819..
- Porta M, Curletto G2, Cipullo D2, Rigault de la Longrais, Trento M, et al. (2014) Estimating the delay between onset and diagnosis of type diabetes from the time course of retinopathy prevalence. Diabetes Care 37(6): 1668-1674.
- Amundson HA, Butcher MK, Gohdes D, Hall TO, Harwell TS, et al. (2009) Translating the Diabetes Prevention Program into practice in the general community: Findings from the Montana Cardiovascular Disease and Diabetes Prevention Program. The Diabetes Educator 35(2): 209-223.
- Diabetes Prevention Program Research Group (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or Metformin. New England Journal of Medicine 346(6): 393-403.
- American Diabetes Association (2010) Standards of medical care in diabetes--2010. Diabetes Care 33 Suppl 1: S11-S61.
- Carson AP, Reynolds K, Fonseca VA, Muntner P (2010) Comparison of A1C and fasting glucose criteria to diagnose diabetes among U.S. adults. Diabetes Care 33(1): 95-97.
- Lipska KJ, De Rekeneire N, Van Ness PH, Johnson KC, Kanaya A, et al. (2010) Identifying dysglycemic states in older adults: implications of the emerging use of hemoglobin A1c. J Clin Endocrinol Metab 95(12): 5289-5295.
- James C, Bullard KM, Rolka DB, Williams DE, Cowie CC, et al. (2011) Implications of alternative definitions of prediabetes for prevalence in U.S. adults. Diabetes Care 34(2): 387-391.
- Cavagnolli G, Pimentel AL, Freitas PA, Gross JL, Camargo JL (2017) Effect of ethnicity on HbA1c levels in individuals without diabetes: Systematic review and meta-analysis. PLoS One 12(2): e0171315.
- Wolffenbuttel BHR, Herman WH, Gross JL, Dharmalingam M, Jiang HH, et al. (2013) Ethnic differences in glycemic markers in patients with type 2 diabetes. Diabetes Care 36(10): 2931-2936.
- Ziemer DC, Kolm P, Weintraub WS, Vaccarino V, Rhee MK, et al. (2010) Glucose-independent, Black - White differences in hemoglobin A1c levels. Ann Intern Med 152(12): 770-777.
- Herman W, Ma Y, Uwaifo G, Haffner S, Kahn SE, et al. (2007) Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 30(10): 2453-2457.
- Cowie CC, Rust KF, Byrd Holt DD, Gregg EW, Ford ES, et al. (2010) Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care 33(3): 562-568.
- Buysschaert M, Medina JL, Buysschaert B, Bergman M (2016) Definitions (and Current Controversies) of diabetes and prediabetes. Curr Diabetes Rev 12(1): 8-13.
- Hivert MF, Christophi C, Jablonski K, Edelstein S, Kahn S, et al. (2018) Genetic ancestry markers and difference in A1c between African- American and White in the Diabetes Prevention Program. J Clin Endocrinol Metab.
- Selvin E, Rawlings AM, Bergenstal RM, Coresh J, Brancati FL (2013) No racial differences in the association of glycated hemoglobin with kidney disease and cardiovascular outcomes. Diabetes Care 36(10): 2295-3001.
- Bloomgarden ZT (2009) A1C: recommendations, debates, and questions. Diabetes Care 32(12): e141-147.
- Dagogo Jack S, Edeoga C. Nyenwe E, Chapp Jumbo E, Wan J (2011) Pathobiology of prediabetes in a biracial cohort (POP-ABC): design and methods. Ethnicity & Disease 21(1): 33-39.
- Herman WH, Cohen RM (2010) Hemoglobin A1c: Teaching a new dog old tricks. Ann Intern Med 152(12): 815-817.
- Williams LB, Sattin RW, Dias J, Garvin JT, Marion L, et al. (2013) Design of a cluster-randomized controlled trial of a diabetes prevention program within African-American churches: The Fit Body and Soul study. Contemporary Clinical Trials 34(2): 336-347.
- Sattin RW, Williams LB, Dias J, Garvin JT, Marion L, et al. (2016) Community Trial of a Faith-Based Lifestyle Intervention to Prevent Diabetes Among African-Americans. J Community Health 41(1): 87-96.
- Cohen R, Smith E (2008) Frequency of HbA1c discordance in estimating blood glucose control. Current Opinion in Clinical Nutrition & Metabolic Care 11(4): 512-517.
- Kirk JK, D Agostino D, RB Jr, Bell RA, Passmore LV, Bonds DE, et al. (2006) Disparities in HbA1c levels between African-American and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes Care 29(9): 2130- 2136.
- Heikes K, Eddy D, Arondekar B, Schlessinger L (2008) Diabetes Risk Calculator: a simple tool for detecting undiagnosed diabetes and prediabetes. Diabetes Care 31(5): 1040-1045.
- Vanderwood KK, Kramer MK, Miller RG, Arena VC, Kriska AM (2015) Evaluation of non-invasive screening measures to identify individuals with prediabetes. Diabetes Res Clin Pract 107(1): 194-201.
- Poltavskiy E, Kim DJ, Bang H (2016) Comparison of screening scores for diabetes and prediabetes. Diabetes Res Clin Pract 118: 146-153.
- Karelis AD, Messier V, Brochu M, Rabasa Lhoret R (2008) Metabolically healthy but obese women: effect of an energy-restricted diet. Diabetologia 51(9): 1752-1754.
- Narayan K, D Williamson D (2010) Prevention of Type 2 Diabetes: Risk Status, Clinic, and Community. Journal of General Internal Medicine 25(2): 154-157.
- Lacy ME, Wellenius GA, Carnethon MR, Loucks EB, Carson AP, et al. (2016) Racial differences in the performance of existing risk prediction models for incident type 2 diabetes: The CARDIA Study. Diabetes Care 39(2): 285-291.
- The ARIC Investigators (1989) The Atherosclerosis Risk in Communities (ARIC) Study: Design and Objectives. American Journal of Epidemiology 129(4): 687-702.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...