Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4579
Research Article(ISSN: 2637-4579) 
Extraction of Starch from Ginger Rhizome (ZingiberOfficinale) Volume 2 - Issue 4
Sudha Melluri and Abbaraju Krishna Sailaja*
- RBVRR Women’s college of pharmacy, Osmania University, India
Received: July 02, 2018; Published: July 09, 2018
Corresponding author: A Krishna Sailaja, Associate professor and Head, RBVRR Women’s college of pharmacy, Osmania university, Hyderabad,India, Tel: 0402756 3065; Email: shailaja1234@rediffmail.com
DOI: 10.32474/OAJBEB.2018.02.000143
Abstract
Zingiberofficinale belonging to family Zingiberaceae is an ancient Indian medicine used in several disorders. The main object behind to isolate starch from the ginger rhizome. Ginger is the rhizome of the plant Zingiberofficinale, Starch is one of the most abundant organic chemicals on earth and it is synthesized in the amyloplasts of seeds, grain, roots and tubers of many plants where it serves as the chemical storage form of energy from the sun. Many drugs commonly used today are herbal origin. Herbal medicine is the oldest form of healthcareknown to mankind. Herbs have been used by all cultures throughout history. Herbal medicine is also in great demand in developed world for primary healthcare because of their efficacy, safety and lesser side effects. The aim of present study is to isolate and Phytochemical screening tests were performed. Zinger officinale rhizomes were extracted by using centrifugation process at 3500rpm. Preliminary Phytochemical screening tests of ginger rhizome extract were performed. Ginger contains a many constituent like starch, fat, Gingerol, volatile oil. And it is used traditionally for dizziness, arthritis, menstrual pain. While isolation of starch from ginger rhizome will be used for pharmacological effect.
Introduction
Since the beginning of human civilization, medicinal plants have been used by mankind for itstherapeutic value. Nature has been a source of medicinal agent for thousands of years and animpressive number of modern drugs have been isolated from natural sources. Many of theseisolations were based on the usage of the agents in the traditional medicine system continues toplay an essential role in health care, with about 80% of the world’s inhabitants relying mainlyon traditional medicines for their primary health care.India has several traditional medical systems, such as Ayurveda and Unani, which hassurvived through more than 3000 years, mainly using plant-based drugs[1,2]. The material medicaof these systems contains a rich heritage of indigenous to herbal practices that have help tosustain the health of most rural people of India. The ancient texts like the Rig Veda (4500-1600BC) and the Atharva Veda mention the use of several plants as medicine. The books on ayurvedic medicine such as Charaka Samhita and Sushruta Samhita refer to the use of morethan 700 herbs[3,4]. According to the World health organization (WHO, 1977) “a medicinal plant” is any plant,which in one or more of its organ contains substances that can be used for the therapeuticpurpose or which, are precursors for the synthesis of useful drugs[5]. This definition distinguishesthose plants whose therapeutic properties and constituents have been established scientificallyand plants that are regarded as medicinal but which have not yet been subjected to a thoroughinvestigation. The term “Herbal drug” determines part/ parts of plant (leaves, seeds, roots,rhizomes) used for preparing medicine[6]. Furthermore, WHO (2001) defines medicinal plant asherbal preparations produced by subjecting plant materials to extraction, purification,concentration or other physical or biological processes which may be produced for immediateconsumption or as a basis for herbal products. Medicinal plants are plants containing inherentactive ingredients used to cure disease or relive pain[7].
Material and Methodology
The materials that are used are fresh ginger rhizomes of about 250gm with required sufficient quantity of (700mL1%W/V) of sodium metabi sulphate and required quantity of water
Extraction Process
This paste was dispersed in (1%of 1g) of sodium Meta bi sulphate in 100ml of distilled water. So that the paste was filtered through a muslin cloth. The suspension was centrifuged at 3500rpm for 10mns to removal of dirt particles to facillate to form cleared supernant and it is decanted and finally the mucilage is scraped off. This following centrifugation process is repeated for four times by keeping with the same rpm and time to get the fine extraction of ginger starch. After getting the fine starch was further died at 60c in hot air oven and finally the starch is weighed and stored[8].
Determination of Swelling Power
The sample was taken (0.1g) of starch and to that 10ml of distilled water was added, and then this mixture was heated in a water bath at 50c for 30mns with continuous shaking. After that the mixture was taken into centrifuge tubes for the balancing take another tube with a distilled water and for centrifugation for 20mns at 1500rpm to facilitate to remove of supernatant and carefully decanted and weight of starch paste was taken to calculate the swelling power by using following formula as follows as:
Swelling power =weight of starch paste̶
Weight of dry starch sample
Determination of Solubility Power
Starch sample containing 0.5g was added to 10ml of distilled water and it is heated for 30mns at 50c in a water bath. This mixture was subjected to centrifuge at 1500rpm for 30mns. In this 5ml of supernatant was decanted to constant weight. So that the solubility was expressed as percentage by following formula as follows as
%percentage solubility =weight of starch paste×100
Weight of sample on dry basis
Gelatinization Temperature
Take 20ml beaker to that add 1gm of starch sample and dissolved in 10ml of distilled water. And it is heated on a hot plate. So that the gelatinization temperature was noted by using thermometer when dispersion is suspended in a starch slurry form[9].
Identification Tests for to Characterise Various Chemical Constituents
Test For Carbohydrates
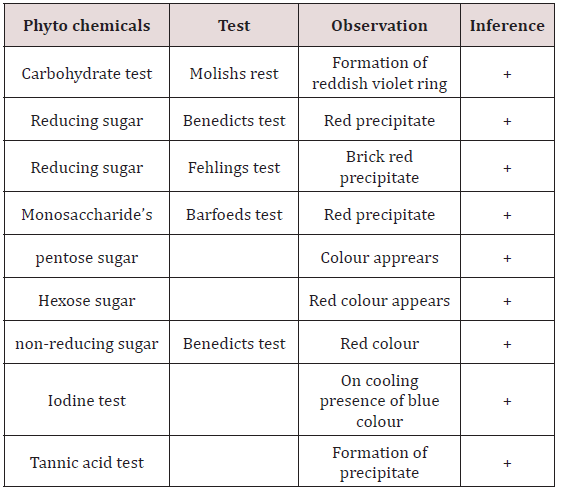
Molishs test: To the 2ml of plant extract add molishs reagent of few drops in a test tube and 2ml of conc.sulphuric acid was added along the sides of test tube the formation reddish violet ring indicates at the junction of two layers that indicates as presence of carbohydrates.
Tests for Reducing Sugar
Benedicts test: To 2ml of benedicts reagent, 1ml of extract was added and allow to warm to stand for 2mns so, that it gets red precipitate and that indicates the presence of sugar.
Fehlings test: Mix the equal volume of (5ml) extract solution to fehlings solution (same volume of fehlings solution A andB) and boil it for few minutes until the appearance ofbrick red precipitate. It indicates the presence of reducing sugar.
Test for monosaccharide’s
Barfoeds test: Take the equal volumes of extact solution and barfoeds reagent. Allow it to heat in water bath for 1-2mns and cool it a side then it gets red precipitate for indication of presence of monosaccharides.
Test for Pentose Sugar
Test for pentose sugar: For the 2ml of test solution add 2ml of HCL and heat for 1-2mns while heating add phloroglucinol then read the colour appears.
Test for hexose sugar: Take water bath to heat 3ml of selwinoffs reagent and add 1ml oftest solution and it is heated upto 2mns until the red colour appears[10].
Test for Non-Reducing Sugar
Benedict’s test: Take 1ml of test solution (extract solution) to that adds few ml of Benedict’s reagent and allowswarming for a few minutes then it gets red precipitate. So, that it indicates presence of sugar (Table 1).
Iodine test: For the 3ml of test solution add few drops of (1-2) iodine solution. Allow it to for few minutes while, boiling absence of blue colour will appear, while cooling presence of blue colour will appear[11].
Tannic acid test: To the 3ml of test solution add 2% of tannic acid then it will get precipitate that indicates of tannins are present.
Results
From the above identification of preliminary screening tests shows the presence of carbohydrates are extracted from the starch of zinger.
Conclusion
The present study the starch was extracted from zinger officinale by using centrifugation process at 3500 rpm for 10mns. And the process is repeated for four times to get the fine starch. This fine starch is carried for the phytochemical screening tests. There that starch that indicates the presence of carbohydrates
References
- Kamal LB (2013) Comparative chemical constituents and antimicrobial activity of normal and organic ginger oils (Zingiber officinale roscoe) 4(1): 259-266.
- Kolawole SA (2013) Comparison of the physicochemical properties of starch from ginger (Zingiber officinale) and maize (Zea mays) 2(11): 71- 75.
- Kokate CK, Gokhale SB, Purohit AP (2009) A textbook of Pharmacognosy, 29th edn, Nirali Prakashan, Pune, India.
- Aguilera Y, Esteban RM, Benítez V, Mollá E, MartínCabrejas MA (2009) Starch, functional properties, and microstructural characteristics in chickpea and lentil as affected by thermal processing. Journal of Agricultural and Food Chemistry 57(22): 10682-10688.
- Araujo Farro PCA, Podadera G, Sobral PJA, Menegalli FC (2010) Development of films based on quinoa (Chenopodium quinoaWilldenow) starch. Carbohydrate Polymers, Baking 81(4): 839-848.
- Devereux S, Shuttle Worth PS, Macquarie DJ, Paradisi F (2011) Isolation and Characterization of Recovered Starch from Industrial Waste water. Journal of Polymers and the Environment 19(4): 971-979.
- M Asaoka, K Okuno, H Fuwa (1985) Effect of environmental temperature at the milky stage on amylose content and fine structure of amylopectin of waxy and non-waxy endosperm starches of rice (Oryza sativa L). Agricultural and Biological Chemistry 49(2): 373-379.
- LJ Zhu, QQ Liu, Y Sang, MH Gu, YC Shi (2010) Underlying reasons for waxy rice flours having different pasting properties. Food Chemistry 120(1): 94-100.
- H Chanapamokkhot, M Thongngam (2007) The chemical and physicochemical properties of sorghum starch and flour. Kasetsart Journal (Natural Science) 41: 343-349.
- I Lindqvist (1979) Cold gelatinization of starch. Starch-Strake 31(6): 195-200.
- HF Zobel (1988) Starch crystal transformations and their industrial importance. Starch-Starke 40(1): 1-7.
Editorial Manager:
Email:
biomedicalengineering@lupinepublishers.com

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...